|
piperonyl butoxide
Insecticide synergist
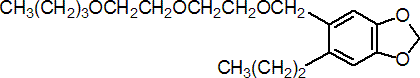
NOMENCLATURE
Common name piperonyl butoxide (BAN; accepted in lieu of a common name by BSI, E-ISO, ESA); piperonyl butoxyde (F-ISO)
IUPAC name 5-[2-(2-butoxyethoxy)ethoxymethyl]-6-propyl-1,3-benzodioxole; 2-(2-butoxyethoxy)ethyl 6-propylpiperonyl ether
Chemical Abstracts name 5-[[2-(2-butoxyethoxy)ethoxy]methyl]-6-propyl-1,3-benzodioxole
Other names PBO CAS RN [51-03-6] Official codes ENT 14 250
PHYSICAL CHEMISTRY
Composition 90% min. piperonyl butoxide. Mol. wt. 338.4 M.f. C19H30O5 Form Colourless liquid; (tech. is a yellow oil). B.p. 180 ºC/1 mmHg (tech.) V.p. 2.0 ´ 10-2 mPa (60 °C) (gas saturation method) KOW logP = 4.75 Henry <2.3 ´ 10-6 Pa m3 mol-1 (calc.) S.g./density 1.060 (20 °C) (ENDQC-4 method) Solubility In water 14.3 mg/l (25 °C). Soluble in all common organic solvents including mineral oils and fluorinated aliphatic hydrocarbons (aerosol propellants). Stability Essentially stable to hydrolysis at pH 5, 7 and 9 in sterile buffers in the dark at 25 °C. Rapidly degraded in aqueous solution (pH 7) in sunlight (DT50 8.4 h). F.p. 140 °C (ASTM D93) Other properties Viscosity 40 cP (25 °C)
COMMERCIALISATION
History Activity as a synergist for pyrethrins described by H. Wachs (Science, 1947, 105, 530). Patents US 2485681; US 2550737 Manufacturers Endura; Prentiss
APPLICATIONS
Biochemistry Inhibits mixed function oxidases (MFO) of the insect so that its natural detoxification system is blocked and the efficacy of applied insecticides is increased. Uses A synergist for the pyrethrins and related insecticides. Formulation types Aerosol; Emulsion; Oil. Selected products: 'Butacide' (Bayer CropScience); mixtures: 'Chinethrin' (+ tetramethrin+ permethrin) (Agro-Chemie); 'Duracide' (+ tetramethrin) (Endura); 'Hash' (+ pyrethrins (pyrethrum)) (Kemio); 'Multi-Fog DTP' (+ tetramethrin+ deltamethrin) (Trithin); 'Pycon' (+ pyrethrins (pyrethrum)) (Agropharm); 'Pyronyl' (+ pyrethrins (pyrethrum)) (Prentiss); 'Synpren fish' (+ rotenone) (Prentiss)
OTHER PRODUCTS
Mixtures: 'Allevol' (+ bioallethrin+ permethrin) (Kemio); 'Amber' (+ tetramethrin+ cypermethrin) (Trithin); 'Asgrow Thirethrin' (+ pyrethrins (pyrethrum)+ endosulfan) (Agriliance); 'Duracide P' (+ tetramethrin+ permethrin) (Endura); 'Hash J25' (+ pyrethrins (pyrethrum)) (aerosol) (Kemio); 'K-obiol' (+ deltamethrin) (Bayer CropScience); 'Metra-Side CTP' (+ tetramethrin+ chlorpyrifos) (Trithin); 'Metrovol' (+ bioallethrin+ permethrin) (Kemio); 'Multi-Fog ATP' (+ tetramethrin+ alpha-cypermethrin) (Trithin); 'NPB' (+ bioallethrin S-cyclopentenyl isomer+ cypermethrin) (Trithin); 'Nusyn-Noxfish' (+ rotenone) (Prentiss); 'Parexan' (+ pyrethrins (pyrethrum)+ S421) (Bayer CropScience); 'PB-Nox' (+ rotenone) (Penick); 'Permanone' (+ permethrin) (Bayer CropScience, Valent Biosciences); 'Pesguard NB' (+ tetramethrin) (Sumitomo); 'Phinco - T 22' (+ tetramethrin+ permethrin) (Vapco); 'Piretrin' (+ pyrethrins (pyrethrum)) (Chemia); 'Pyrenone' (+ pyrethrins (pyrethrum)) (Bayer CropScience); 'Scourge' (+ resmethrin) (Bayer CropScience); 'Stald-Chok' (+ resmethrin) (Aeropak); 'Tornado-Forte+' (+ tetramethrin+ lambda-cyhalothrin) (public health) (Vapco); 'ULV500' (+ tetramethrin+ phenothrin [(1R)-trans- isomer]) (Trithin) Discontinued products mixtures: 'Duracide Cyp' * (+ tetramethrin+ cypermethrin) (Endura); 'Pyrotex' * (+ allethrin [(1R)- isomers]) (Protex SA); 'Supercord' * (+ alpha-cypermethrin) (Shell)
ANALYSIS
Product analysis by glc with FID (CIPAC Handbook, 1985, 1C, 2190, 2209; ibid., 1998, H, 239; Br. Pharmacopoeia Vet., 1977, p. 64; AOAC Methods, 17th Ed., 982.02*). Residues determined by colorimetry of a derivative (Anal. Methods Pestic. Plant Growth Regul., 1972, 6, 458; AOAC Methods, 17th Ed., 960.43*; Pestic. Anal. Man., 1979, II; Man. Pestic. Residue Anal., 1987, I, 6, S19; Anal. Methods Residues Pestic., 1988, Part II; R. T. Krause & E. M. August, J. Assoc. Off. Anal. Chem., 1983, 66, 234, 1018).
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 74, 76, 92 (see part 2 of the Bibliography). IARC ref. 30 class 3 Oral Acute oral LD50 for rats and rabbits c. 7500 mg/kg. Skin and eye Acute percutaneous LD50 for rats >7950, rabbits 1880 mg/kg. Not irritant to eyes or skin; not a skin sensitiser. Inhalation LC50 for rats >5.9 mg/l. NOEL (2 y) for mice and rats 30 mg/kg b.w. daily; (1 y) for dogs 16 mg/kg b.w. daily. ADI (JMPR) 0.2 mg/kg b.w. [1995, 2001]. Other Not teratogenic, mutagenic or carcinogenic. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250 mg/kg. Fish LC50 (24 h) for carp 5.3 mg/l. Daphnia LC50 (24 h) 2.95 mg/l. Algae EC50 (cell volume) for Chlorella fusca 44 mmol/l (Pestic. Sci., 47, 337 (1996)). Bees LD50 >25 mg/bee.
ENVIRONMENTAL FATE
Animals In mammals (and also in insects), oxidative attack on the carbon atom of the methylenedioxy group leads to the formation of the dihydroxyphenyl compound. Oxidative degradation of the side-chain also occurs. Elimination is as the glucoside or amino acid derivative. Soil/Environment DT50 for aerobic soil metabolism c. 14 d. Koc 399-830. Although mobile in sandy soil, it is not expected to leach under outdoor conditions, where rapid degradation occurs. Degradation in soil or water is mainly via oxidation of the butyl side-chain to form methylenedioxypropylbenzyl alcohol followed by the corresponding aldehyde, ultimately with mineralisation to CO2; there is no accumulation of metabolites.
|