|
tebupirimfos
Insecticide
IRAC 1B; organophosphate
NOMENCLATURE
Common name tebupirimfos (BSI, pa-ISO)
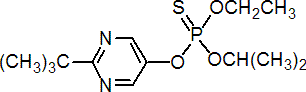
IUPAC name O-(2-tert-butylpyrimidin-5-yl) O-ethyl O-isopropyl phosphorothioate
Chemical Abstracts name O-[2-(1,1-dimethylethyl)-5-pyrimidinyl] O-ethyl O-(1-methylethyl) phosphorothioate
Other names phostebupirim CAS RN [96182-53-5] Development codes BAY MAT 7484 (Bayer)
PHYSICAL CHEMISTRY
Mol. wt. 318.4 M.f. C13H23N2O3PS Form Colourless to amber crystals. B.p. 135 ºC/1.5 mmHg; 152 °C/760 mmHg V.p. 5 mPa (20 °C) Henry 2.89 ´ 10-1 Pa m3 mol-1 (calc.) Solubility In water 5.5 mg/l (20 ºC, pH 7). Soluble in most organic solvents, e.g. alcohols, ketones and toluene. Stability Hydrolysed under alkaline conditions.
COMMERCIALISATION
History Reported by J. Hartwig et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1992, 1, 35). Introduced by Bayer Corp. in 1995. Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Insecticide with contact action and good residual activity. Uses Control of soil insects, especially Diabrotica species, wireworms and Diptera maggots. Formulation types GR. Selected products: mixtures: 'Aztec' (+ cyfluthrin) (Amvac, Bayer CropScience, Makhteshim-Agan)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 2.9-3.6, female rats 1.3-1.8, male mice 14.0, female mice 9.3 mg/kg. Skin and eye Acute percutaneous LD50 for male rats 31.0, female rats 9.4 mg/kg. Inhalation LC50 (4 h) for male rats c. 82, female rats c. 36 mg/m3 air (aerosol). NOEL (2 y) for rats 0.3, mice 0.3, dogs 0.7 mg/kg diet. ADI 0.0002 mg/kg b.w. Other Non-mutagenic and non-teratogenic; no evidence of carcinogenic potential. Toxicity class WHO (a.i.) Ia
ECOTOXICOLOGY
Birds LD50 for bobwhite quail 20.3 mg/kg. LC50 (5 d) for mallard ducks 577, bobwhite quail 191 mg/kg. Fish LC50 (96 h) for rainbow trout 2250, golden orfe 2550 mg/kg. Daphnia LC50 (96 h) 0.078 mg/l. Algae ErC50 (96 h, 23 °C) for Scenedesmus subspicatus 1.8 mg/l.
ENVIRONMENTAL FATE
EHC 63 (WHO, 1986; a general review of organophosphorus insecticides).
|