|
tebufenpyrad
Acaricide
IRAC 21; METI
NOMENCLATURE
Common name tebufenpyrad (BSI, draft ISO)
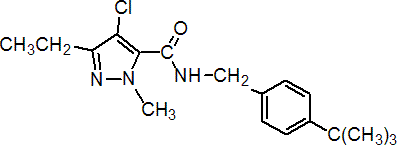
IUPAC name N-(4-tert-butylbenzyl)-4-chloro-3-ethyl-1-methylpyrazole-5-carboxamide
Chemical Abstracts name 4-chloro-N[[4-(1,1-dimethylethyl)phenyl]methyl]-3-ethyl-1-methyl-1H-pyrazole-5-carboxamide
CAS RN [119168-77-3] Development codes AC 801757 (Cyanamid); MK-239 (Mitsubishi Kasei)
PHYSICAL CHEMISTRY
Mol. wt. 333.9 M.f. C18H24ClN3O Form Colourless crystals. M.p. 61-62 ºC V.p. <1 ´ 10-2 mPa (25 ºC) KOW logP = 5.04 (25 ºC) Henry <1.25 ´ 10-3 Pa m3 mol-1 (calc.) S.g./density 1.0214 Solubility In water 2.8 mg/l (25 ºC). Soluble in acetone, methanol, chloroform, acetonitrile, hexane, and benzene. Stability Stable to hydrolysis, DT50 >28 d (pH 5, 7 and 9).
COMMERCIALISATION
History Discovered by Mitsubishi Kasei (now Mitsubishi Chemical Corp., who sold their agrochemical business to Nihon Nohyaku Co., Ltd in 2002) and developed in collaboration with American Cyanamid Co. (now BASF AG). Registered in Japan in 1993. Patents US 4950668; EP 289879.
APPLICATIONS
Biochemistry Mitochondrial respiration inhibitor. Acts as an inhibitor of the electron transport chain at Site I. Does not act as a specific muscle or nerve poison. Mode of action Non-systemic acaricide active by contact and ingestion. Exhibits translaminar movement following application to leaves, and thus inhibits the development of mite eggs oviposited on the undersides of leaves. Uses Control of all stages of Tetranychus, Panonychus, Oligonychus, and Eotetranychus spp. on top fruit, vines, citrus, vegetables, hops, ornamentals, melons, and cotton. Applied at 50-200 g/ha. Formulation types EC; EW; WG; WP. Selected products: 'Comanch? (BASF); 'Masa? (BASF); 'Oscar' (BASF)
OTHER PRODUCTS
'Acarifas' (BASF); 'Pyranica' (Nihon Nohyaku)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 595, female rats 997, male mice 224, female mice 210 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin; slightly irritating to eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 for male rats 2660, female rats >3090 mg/m3. Other No mutagenic effects were observed in the following tests; Ames, mammalian micronucleus, Drosophila wing spot, in vitro cultured human lymphocytes, in vivo bone marrow erythrocytes, unscheduled DNA synthesis, and CHO/HGPRT. Toxicity class WHO (a.i.) III EC classification (Xn; R20/22)
ECOTOXICOLOGY
Birds Acute oral LD50 for mallard ducks >2000 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >5000 mg/kg diet. Fish LC50 (48 h) for carp 0.073 mg/l. Daphnia LC50 (3 h) 1.2 mg/l. Bees Low toxicity to honeybees.
ENVIRONMENTAL FATE
Animals Metabolite is N-[4-(1-hydroxymethyl-1-methylethyl)benzyl]-4-chloro-3-(1-hydroxyethyl)-1-methylpyrazole-5-carboxamide. Plants As for animals. Soil/Environment Aerobic degradation occurs in soil, DT50 20-30 d. Koc 1380-4930.
|