|
sulfosulfuron
Herbicide
HRAC B WSSA 2; sulfonylurea
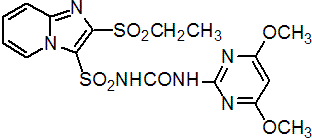
NOMENCLATURE
Common name sulfosulfuron (BSI, pa ISO)
IUPAC name 1-(4,6-dimethoxypyrimidin-2-yl)-3-(2-ethylsulfonylimidazo[1,2-a]pyridin-3-yl)sulfonylurea
Chemical Abstracts name N-[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]-2-(ethylsulfonyl)imidazo[1,2-a]pyridine-3-sulfonamide
CAS RN [141776-32-1] Development codes MON 37500 (Monsanto); TKM 19; MON 37588 (Monsanto) (for WG formulation)
PHYSICAL CHEMISTRY
Mol. wt. 470.48 M.f. C16H18N6O7S2 Form White, odourless solid. M.p. 201.1-201.7 °C V.p. 8.81 ´ 10-8 mPa (25 °C) KOW logP <1 (pH 5, 9) Henry 8.15 ´ 10-7 (pH 5), 8.83 ´ 10-9 (pH 7), 2.97 ´ 10-8 (pH 9) (calc.) S.g./density 1.5185 (20 °C) Solubility In water 17.6 (pH 5), 1627 (pH 7), 482 (pH 9) (all mg/l, 20 °C). In acetone 0.71, methanol 0.33, ethyl acetate 1.01, dichloromethane 4.35, xylene 0.16, heptane <0.01 (all g/l, 20 °C). Stability Stable <54 °C for 14 d. pKa 3.51 (20 °C)
COMMERCIALISATION
History Discovered by Takeda Chemical Industries, Ltd (now Sumitomo Chemical Takeda Agro Company Ltd). Reported by S. K. Parrish et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1995, 1, 57). Developed jointly by Monsanto Co. and Takeda and introduced in 1997. Manufacturers Sumitomo Chemical Takeda
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity derives from rapid metabolism in the crop. Metabolic basis of selectivity in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry, 6, 9-14 (1995)). Mode of action Systemic herbicide, absorbed by the root system and/or leaf surface, and translocated to symplast and apoplast. Uses Has demonstrated activity for the control of annual broad-leaved weeds and grass weeds in cereals (wheat), at 10-35 g/ha. Phytotoxicity Barley and oats are sensitive. Tolerance of durum wheat is variety-specific. Formulation types WG. Compatibility Not compatible with fertiliser solutions with pH £5, nor with non-ionic surfactants or other additives that alter the pH of the spray solution below pH 5. Not compatible with malathion. Crop injury may result if applied within 60 days of crop emergence where an organophosphate insecticide has been applied as an in-furrow treatment. Selected products: 'Maverick' (wheat) (Monsanto); 'Monitor' (wheat) (Monsanto); 'Outrider' (non-crop land) (Monsanto)
OTHER PRODUCTS
'Anthem' (Monsanto); 'Apyros' (Monsanto, Sumitomo Chemical Takeda); 'Leader' (Monsanto); 'Monza' (Monsanto); 'Sundance' (Monsanto)
ANALYSIS
Methods for sulfonylurea residues in crops, soil and water reviewed (A. C. Barefoot et al., Proc. Br. Crop Prot. Conf. - Weeds, 1995, 2, 707).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg. Not a skin irritant; moderate eye irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation Practically non-toxic. ADI 0.24 mg/kg. Toxicity class EPA (formulation) III
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail and mallard ducks >2250 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5620 ppm. Fish LC50 (96 h) for rainbow trout >95, carp >91, bluegill sunfish >96, sheepshead minnow >101 mg/l. Daphnia EC50 (48 h) >96 mg/l. Algae EbC50 (3 d) for green algae (Selenastrum capricornutum) 0.221 mg/l, ErC50 (3 d) 0.669 mg/l; EC50 (5 d) for blue-green algae (Anabaena flos-aquae) 0.77 mg/l. Other aquatic spp. IC50 (14 d) for Lemna gibba (G3) >1.0 mg/l. Bees LD50 (oral) >30 mg/bee; (dermal) >25 mg/bee. Worms LC50 >848 mg/kg. Other beneficial spp. Classified as harmless to Bembidion tetracolum, Pardosa spp., Typhlodromus pyri, Aphidius rhopalosiphi.
ENVIRONMENTAL FATE
Cleavage of the sulfonylurea linkage in soil is a major pathway of metabolism, with oxidative demethylation playing a significant role in some matrices. Animals Sulfosulfuron was eliminated rapidly from rats, with limited metabolism and negligible residues in tissues. O-Demethylation to yield desmethyl sulfosulfuron and ring-hydroxylation on the pyrimidine ring were the primary metabolic pathways. Rapid elimination was also observed in livestock. Negligible transfer and/or retention of sulfosulfuron residues to milk, eggs, organs and tissues were observed in goats and hens. Plants Residues in wheat grain were negligible. The major component in wheat forage and straw from the post-emergent treatment was unmetabolised sulfosulfuron. The major metabolite, a sulfonamide, resulted from cleavage of the sulfonylurea bridge. Minor metabolites resulted from oxidative demethylation to yield desmethyl sulfosulfuron and the ring-opened guanidine analogue. Minimal uptake was observed in rotation crops, with the major metabolite being free and conjugated sulfonamide. Soil/Environment The primary degradation pathway in soil is the hydrolytic cleavage of the sulfonylurea linkage to yield the corresponding sulfonamide and dimethoxypyrimidinamine. DT50 (lab.) 32 d (silt loam, pH 7.6, 0.8% o.m.), 35 d (sandy loam, pH 6.8, 1.6% o.m.), 53 d (loamy sand, pH 5.8, 3.9% o.m.); DT50 was longer in some other soils. Photodegradation is also a mode of environmental dissipation, DT50 3 d. In field studies at eleven sites in Europe, mean DT50 after application to bare soil 24 d (range 11-47 d); mean DT90 261 d. Despite rapid degradation, rotational injury to sensitive crops can be expected; see S. K. Parrish et al., Proc. Br. Crop Prot. Conf. - Weeds, 1995, 1, 667. Degradation in water/sediment system was fairly rapid; DT50 32 d for river (pH 7.0, 1.7% o.m.), 20 d for pond (pH 7.0, 2.9% o.m.) systems (both 20 °C). In the water phase, DT50 19.5 d for river, 16 d for pond. Mobility of sulfosulfuron was limited, based on the results of field dissipation studies and an EU lysimeter study; sulfosulfuron mean concentrations in the lysimeter leachate were <0.01 mg/l over a 3 y study.
|