|
sulcotrione
Herbicide
HRAC F2 WSSA 28; triketone
NOMENCLATURE
Common name sulcotrione (BSI, pa E-ISO)
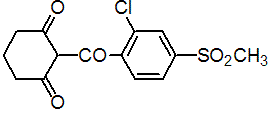
IUPAC name 2-(2-chloro-4-mesylbenzoyl)cyclohexane-1,3-dione
Chemical Abstracts name 2-[2-chloro-4-(methylsulfonyl)benzoyl]-1,3-cyclohexanedione
CAS RN [99105-77-8] Development codes ICIA0051 (ICI); SC0051
PHYSICAL CHEMISTRY
Composition Tech. material is 90% pure. Mol. wt. 328.8 M.f. C14H13ClO5S Form White solid; (tech. is a light tan solid). M.p. 139 ºC; (tech., 131-139 ºC) V.p. 5 ´ 10-3 mPa (25 ºC) KOW logP <0 (unspecified pH) Henry 9.96 ´ 10-6 Pa m3 mol-1 (calc.) Solubility In water 165 mg/l (25 ºC). Soluble in acetone and chlorobenzene. Stability Stable in water, with or without exposure to sunlight. Thermostable up to 80 ºC. pKa 3.13 (23 °C)
COMMERCIALISATION
History Reported by J. M. Beraud et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1991, 1, 51). Introduced by ICI (now Syngenta AG) and first marketed in 1991. Rights in some territories divested to Bayer AG, in 2000.
APPLICATIONS
Biochemistry p-Hydroxyphenyl pyruvate dioxygenase inhibitor. Mode of action Herbicide, absorbed predominantly by the leaves, but also by the roots. Uses Control of broad-leaved weeds and grasses post-emergence in maize and sugar cane, at 200-300 g/ha. Formulation types SC. Selected products: 'Galleon' (Bayer CropScience); 'Mikado' (Bayer CropScience)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >4000 mg/kg. Low rate of absorption through the skin; non-irritating to skin; mild eye irritant (rabbits). Strong skin sensitiser (guinea pigs). Inhalation LC50 (4 h) >1.6 mg/l. NOEL (2 y) for rats 100 ppm (0.5 mg/kg b.w. daily). ADI 0.005 mg/kg. Other Non-teratogenic in rats and rabbits. Non-genotoxic as demonstrated in vivo.
ECOTOXICOLOGY
Birds Dietary LC50 for bobwhite quail and mallard ducks >5620 mg/kg. Fish LC50 (96 h) for rainbow trout 227, mirror carp 240 mg/l. Daphnia LC50 (48 h) >200 mg/l. Algae EC50 (96 h) for Selenastrum capricornutum 1.2 mg/l. Bees Low toxicity to bees, both by topical and oral application; LD50 >200 mg/bee. Worms LC50 (14 d) >1000 mg/kg soil.
ENVIRONMENTAL FATE
Animals Rapidly excreted in the urine, the major metabolite being 4-hydroxysulcotrione. Plants Deactivated by the formation of 2-chloro-4-methylsulfonylbenzoic acid. Soil/Environment Rapidly degraded in soil; lab. DT50 15-74 d; field DT50 1-11 d. The major metabolite is 2-chloro-4-methylsulfonylbenzoic acid. Koc 44 (high pH, sandy clay loam) to 940 (low pH sand). No adverse effects on soil micro-organisms.
|