|
spiroxamine
Fungicide
FRAC 5, G2; morpholine: spiroketalamine
NOMENCLATURE
Common name spiroxamine (BSI, pa ISO)
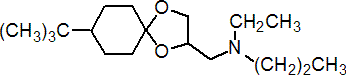
IUPAC name 8-tert-butyl-1,4-dioxaspiro[4.5]decan-2-ylmethyl(ethyl)(propyl)amine
Chemical Abstracts name 8-(1,1-dimethylethyl)-N-ethyl-N-propyl-1,4-dioxaspiro[4,5]decane-2-methanamine
CAS RN [118134-30-8] Development codes KWG 4168 (Bayer)
PHYSICAL CHEMISTRY
Composition Comprises 2 diastereoisomers, A and B, in the proportions 49-56% and 51-44%, respectively. Mol. wt. 297.5 M.f. C18H35NO2 Form Faintly yellowish liquid; (tech. is a light brown, oily liquid). M.p. <-170 °C (separate diastereoisomers, spiroxamine A, spiroxamine B, and tech. mixture of both diastereoisomers) B.p. Decomp. c. 120 °C V.p. A: 9.7 mPa (20 °C); B: 17 mPa (25 °C) (both extrapolated) KOW A: logP = 2.79; B: logP = 2.92 (unstated pH) Henry A: 2.5 ´ 10-3; B: 5.0 ´ 10-3 (both in Pa m3 mol-1, 20 °C, calc.) S.g./density A and B both 0.930 (20 °C) Solubility In water, mixture of A and B: >200 ´ 103 mg/l (pH 3, 20 °C); A: 470 (pH 7), 14 (pH 9); B: 340 (pH 7), 10 (pH 9) (both diastereoisomers in mg/l, 20 °C). Stability Stable to hydrolysis and photodegradation; provisional photolytic DT50 50.5 d (25 °C). pKa 6.9, base F.p. 147 °C
COMMERCIALISATION
History Discovered in 1987. Reported by S. Dutzmann et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1996, 1, 47).First sold in 1997. Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Sterol biosynthesis inhibitor, acting mainly by inhibition of D14-reductase. Mode of action Protective, curative and eradicative systemic fungicide. Readily penetrates into the leaf tissue, followed by acropetal translocation to the leaf tip. Uniformly distributed within the whole leaf. Uses Control of powdery mildew in cereals (Erysiphe graminis), at 500-750 g/ha, and in grapes (Uncinula necator), at 400 g/ha. Also gives good control of rusts (Rhynchosporium and Pyrenophora teres), together with certain side-effects against Septoria diseases. Penetration studies have shown that tank mixes of spiroxamine and triazoles can positively influence the uptake of triazoles in plants. Formulation types EC; EW. Selected products: 'Impulse' (Bayer CropScience); 'Prosper' (Bayer CropScience); mixtures: 'Pronto Plus' (+ tebuconazole) (spray, Germany) (Bayer CropScience)
OTHER PRODUCTS
'Aquarelle' (Bayer CropScience); 'Hoggar' (Bayer CropScience); 'Neon' (Bayer CropScience); 'Torch' (Bayer CropScience); 'Zenon' (Bayer CropScience) mixtures: 'Array' (+ tebuconazole) (spray, UK) (Bayer CropScience); 'Beam' (+ tebuconazole) (spray, UK) (Bayer CropScience); 'Bronze' (+ tebuconazole) (spray, UK) (Bayer CropScience); 'Buster' (+ tebuconazole) (spray, France) (Bayer CropScience); 'Draco' (+ tebuconazole) (spray, UK) (Bayer CropScience); 'Falcon' (+ tebuconazole+ triadimenol) (spray) (Bayer CropScience); 'Hudson' (+ tebuconazole) (spray, France) (Bayer CropScience); 'Orca' (+ tebuconazole) (spray, Belgium, France) (Bayer CropScience); 'Sage' (+ tebuconazole) (spray, UK) (Bayer CropScience)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats c. 595, female rats 500-560 mg/kg. Skin and eye Acute percutaneous LD50 for male rats >1600, female rats c. 1068 mg/kg b.w. Severe skin irritant; not an eye irritant (rabbits). Inhalation LC50 (4 h) for male rats c. 2772, female rats c. 1982 mg/m3. NOEL (2 y) for rats 70, mice 160 mg/kg diet; (1 y) for dogs 75 mg/kg diet. ADI 0.025 mg/kg b.w. Other Not genotoxic; no specific effects on reproduction. Toxicity class WHO (a.i.) II; EPA (formulation) II EC classification Xn; R20/21/22| Xi; R38| R43| N; R50, R53
ECOTOXICOLOGY
Birds LD50 for bobwhite quail 565 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5000 mg/kg. Fish LC50 (96 h, static) for rainbow trout 18.5, bluegill sunfish 7.13 mg/l. Algae ErC50 (96 h) for Scenedesmus subspicatus 0.012, Pseudokirchneriella subcapitata 0.0194 mg/l. Bees LD50 (oral) >100 µg/bee; (contact) 4.2 µg/bee. Worms LC50 ³1000 mg/kg dry wt. substrate.
ENVIRONMENTAL FATE
Animals Biokinetics and metabolism studies in rats showed a high degree of absorption of radioactivity followed by fast elimination from the body (>97 % within 48 h after oral administration). The radioactivity was readily distributed from the plasma into peripheral compartments. The main metabolite in all dose groups was the compound oxidised to the carboxylic acid in the t-butyl moiety. In dairy goats and laying hens, the total radioactive residue of spiroxamine in tissues, organs and milk was relatively low due to fast elimination; metabolism proceeds either via oxidation of the t-butyl moiety to yield the carboxylic acid compound or via desalkylation of the amino group resulting in the des-ethyl and des-propyl derivatives of spiroxamine. For animal tissues, the carboxylic acid was defined as the relevant residue. Plants Extensively metabolised in spring wheat and grapes. Oxidation occurred preferentially in the tertiary amine group (formation of the N-oxide) and also to a minor extent in the t-butyl group (e.g. the hydroxy compound). Some metabolites were formed by dealkylation. Based on the results of the metabolism in plants, the residue definition includes the parent compound as well as all metabolites containing the 4-t-butylcyclohexyl moiety. Soil/Environment Readily degraded in soil, ultimately to CO2; oxidation on the t-butyl moiety and des-alkylation of the amine are the primary reaction steps. The des-alkylated compounds were either further oxidised to the corresponding acids or further degraded to a ketone metabolite. Soil DT50 (lab. and field) in the range 35-64 d. The relevant residue in soil and air is the parent compound. Relatively stable to hydrolysis at pH 9; direct photodegradation in water is not a significant means of degradation. In water/sediment studies, spiroxamine bound rapidly to the sediment; DT50 in the supernatant water 12-13 h. Thoroughly degraded in the water/sediment systems, ultimately to CO2. In water, the relevant residue for quantitation, besides the parent compound, is the N-oxide only.
|