|
pyridaphenthion
Insecticide, acaricide
IRAC 1B; organophosphate
NOMENCLATURE
Common name pyridaphenthion (JMAF)
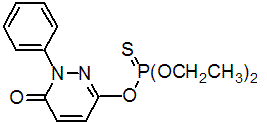
IUPAC name O-(1,6-dihydro-6-oxo-1-phenylpyridazin-3-yl) O,O-diethyl phosphorothioate
Chemical Abstracts name O-(1,6-dihydro-6-oxo-1-phenyl-3-pyridazinyl) O,O-diethyl phosphorothioate
CAS RN [119-12-0] Development codes NC-250; CL 12503 (Cyanamid)
PHYSICAL CHEMISTRY
Mol. wt. 340.3 M.f. C14H17N2O4PS Form Pale yellow solid. M.p. 54.5-56.0 ºC V.p. 0.00147 mPa (20 ºC) KOW logP = 3.2 Henry 5.00 ´ 10-6 Pa m3 mol-1 (calc.) S.g./density 1.325 (20 ºC) Solubility In water 100 ppm (20 °C). In acetone 3.77, methanol 2.66 (both in kg/kg, 21 ºC); in diethyl ether 1.01 kg/kg (25 ºC).
COMMERCIALISATION
History Insecticide introduced in Japan (1974) by Mitsui Toatsu Chemicals, Inc. (now Mitsui Chemicals, Inc.). Manufacturers Mitsui
APPLICATIONS
Biochemistry Cholinesterase inhibitor. Mode of action Insecticide and acaricide with contact and stomach action. Uses Control of sucking and chewing insects (e.g. stem borers, leaf rollers, leafhoppers, planthoppers, leaf beetles, skippers, grasshoppers, thrips, aphids, sawflies, lace bugs, caterpillars, onion flies, bean seed flies, etc.) and spider mites on paddy rice, vegetables, fruit, and ornamentals. Also effective against Isoptera in public health. Formulation types DP; EC; UL; WP. Compatibility Incompatible with alkaline products. Selected products: 'Ofunack' (Mitsui); 'Oreste' (Sipcam Phyteurop)
ANALYSIS
Product and residue analysis by glc.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 769, female rats 850, mice 459, dogs >12 000 mg/kg. Skin and eye Acute percutaneous LD50 for male rats 2300, female rats 2100 mg/kg. Non-irritating to skin (rabbits). Inhalation LC50 (4 h) for rats >1133.3 mg/m3. Other In teratogenicity, mutagenicity, carcinogenicity, and multigeneration chronic toxicity studies on rats, no adverse effects were observed. Toxicity class WHO (a.i.) III
ECOTOXICOLOGY
Birds Acute oral LD50 for Japanese quail 68 mg/kg. Fish TLm (48 h) for carp 12 ppm. Daphnia TLm (3 h) 0.02 mg/l. Bees LD50 0.08 mg/bee.
ENVIRONMENTAL FATE
EHC 63 (WHO, 1986; a general review of organophosphorus insecticides). Animals In mice and rats, the parent compound, O-ethyl-O-(3-oxo-2-phenyl-2H-pyridazine-6-yl) phosphorothioate and the corresponding phosphate are found. Plants In rice, phenyl maleic hydrazide (PMH), O,O-diethyl thiophosphoric acid, and PMH glycoside are formed. Soil/Environment Soil DT50 11-24 d.
|