|
pretilachlor
Herbicide
HRAC K3 WSSA 15; chloroacetamide
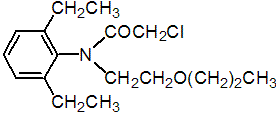
NOMENCLATURE
Common name pretilachlor (BSI, E-ISO); prétilachlore ((m) F-ISO)
IUPAC name 2-chloro-2',6'-diethyl-N-(2-propoxyethyl)acetanilide
Chemical Abstracts name 2-chloro-N-(2,6-diethylphenyl)-N-(2-propoxyethyl)acetamide
CAS RN [51218-49-6] Development codes CGA 26 423 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Mol. wt. 311.9 M.f. C17H26ClNO2 Form Colourless liquid. B.p. 135 ºC/0.001 mmHg V.p. 0.133 mPa (20 ºC) KOW logP = 4.08 (shake flask) Henry 8.1 ´ 10-4 Pa m3 mol-1 S.g./density 1.076 (20 ºC) Solubility In water 50 mg/l (20 ºC). Very soluble in benzene, hexane, methanol and dichloromethane. Stability Relatively stable to hydrolysis; DT50 (calc.) (20 ºC) >200 d (pH 1-9), 14 d (pH 13). F.p. 129 °C (EEC A9)
COMMERCIALISATION
History Introduced by Ciba-Geigy AG (now Syngenta AG) in 1988. Patents BE 800471; GB 1438311; GB 1438312 Manufacturers Sharda; Syngenta
APPLICATIONS
Biochemistry Cell division inhibitor; more recent research suggests chloroacetamides may inhibit synthesis of very long chain fatty acids (J. Schmalfuss et al., Abstr. Meeting WSSA, Toronto, 40, 117-118, 2000; P. Böger, Abstr. III Int. Weed Control Congr., Brazil 2000). Mode of action Selective herbicide. It is taken up readily by the hypocotyls, mesocotyls and coleoptiles, and, to a lesser extent, by the roots of germinating weeds. Uses Herbicide effective against main annual grasses, broad-leaved weeds and sedges in transplanted and seeded rice, at 1-1.25 kg/ha. Phytotoxicity Applied alone, pretilachlor will cause injury to direct-seeded rice. Formulation types EC; GR. Selected products: 'Rifit' (transplanted rice) (Syngenta); 'Solnet' (transplanted rice) (Syngenta); 'Gorbo' (Sumitomo Chemical Takeda); mixtures: 'Sofit' (+ fenclorim) (direct-sown rice) (Syngenta); 'Sing' (+ pyributicarb) (Sankyo Agro); 'Sparkstar G' (+ pyrazosulfuron-ethyl+ dimethametryn+ esprocarb) (Nissan, Syngenta)
OTHER PRODUCTS
Mixtures: 'Hayate' (+ daimuron+ dimethametryn+ imazosulfuron) (Sumitomo Chemical Takeda); 'Kusa Kont' (+ benzobicyclon) (SDS Biotech KK, Syngenta, Sankyo Agro); 'Kusahope D' (+ pyrazolynate+ dimethametryn) (Sankyo Agro); 'Kusanain' (+ pyributicarb+ bensulfuron-methyl+ dimethametryn) (DuPont); 'Pulgman' (+ daimuron+ ethoxysulfuron) (Bayer CropScience); 'Sheriff' (+ cyhalofop-butyl+ dimethametryn+ imazosulfuron) (Otsuka, Sumitomo Chemical Takeda); 'Slasher' (+ pyrazolynate+ bromobutide+ dimethametryn) (Sankyo Agro); 'Topran' (+ pyrazolynate+ ethoxysulfuron) (Bayer CropScience); 'Urifos' (+ pyrazolynate+ benfuresate+ dimethametryn) (Sankyo Agro); 'Wydori A' (+ azimsulfuron+ bensulfuron-methyl+ dimethametryn) (Nihon Nohyaku) Discontinued products: 'Erijan' * (Ciba) mixtures: 'Kusahope' * (+ pyrazolynate) (Sankyo); 'Tabijin A' * (+ pyriminobac-methyl+ azimsulfuron+ bensulfuron-methyl+ cyhalofop-butyl) (Kumiai)
ANALYSIS
Product analysis by glc. Residues determined by glc with MCD or TID. Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 6099 mg/kg. Skin and eye Acute percutaneous LD50 for rats >3100 mg/kg. Moderate irritant to skin; non-irritant to eyes (rabbits). Inhalation LC50 (4 h) for rats >2.8 mg/l air. NOEL (2 y) for rats 30, mice 300 ppm; (0.5 y) for dogs 300 ppm. ADI 0.018 mg/kg. Toxicity class WHO (a.i.) U EC classification (R38, R43)
ECOTOXICOLOGY
Birds Non-toxic to birds; LD50 for Japanese quail >10 000 mg/kg. LC50 for Japanese quail >1000 ppm. Fish LC50 (96 h) for rainbow trout 0.9, catfish 2.7, crucian carp 2.3 mg/l. Daphnia LC50 13 mg/l. Algae EC50 for Selenastrum capricornutum 0.002 mg/l. Bees LD50 (contact) 93 mg/bee.
ENVIRONMENTAL FATE
Animals Substitution of the chlorine atom for glutathione to form a conjugate. Cleavage of the ether bond to yield an ethyl alcohol derivative. Both metabolites are susceptible to further degradation. Plants Substitution of the chlorine atom to form a conjugate. Cleavage of the ether bond to yield an ethyl alcohol derivative. Hydrolytic and reductive removal of the chlorine atom. Soil/Environment Applied to paddy water, pretilachlor disappeared from the water by adsorption to the soil, where it is rapidly degraded under practical conditions, median DT50 (lab.) 30 d. Due to strong soil adsorption, unlikely to leach.
|