|
pencycuron
Fungicide
FRAC 20, B4; phenylurea fungicide
NOMENCLATURE
Common name pencycuron (BSI, draft E-ISO, (m) draft F-ISO)
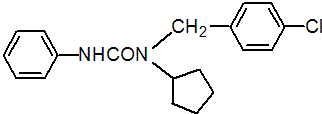
IUPAC name 1-(4-chlorobenzyl)-1-cyclopentyl-3-phenylurea
Chemical Abstracts name N-[(4-chlorophenyl)methyl]-N-cyclopentyl-N'-phenylurea
CAS RN [66063-05-6] EEC no. 266-096-3 Development codes NTN 19 701 (Bayer)
PHYSICAL CHEMISTRY
Mol. wt. 328.8 M.f. C19H21ClN2O Form Colourless, odourless crystals. M.p. 128 °C (modification A); 132 °C (modification B) V.p. 5 ´ 10-7 mPa (20 ºC, extrapolated) KOW logP = 4.68 (20 °C) Henry 5 ´ 10-7 Pa m3 mol-1 (20 °C) S.g./density 1.22 (20 °C) Solubility In water 0.3 mg/l (20 ºC). In dichloromethane 270, toluene 20, n-hexane 0.12 (all in g/l, 20 ºC). Stability Hydrolysis DT50 64-302 d (25 ºC). Photodegrades in water and on soil surfaces.
COMMERCIALISATION
History Fungicide reported by P-E. Frohberger & F. K. Grossman (Mitt. Biol. Bundesanst. Land-Forstwirtsch., Berlin-Dahlem, 1981, 203, 230). Introduced by Bayer AG and first marketed in 1984. Patents BE 856922; DE 2732257 Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Cell division inhibitor. Mode of action Non-systemic fungicide with protective action. Uses Can be used as a foliar spray and dust application, a seed treatment, or by soil incorporation. Control of diseases caused by Rhizoctonia solani and Pellicularia spp. in potatoes (15-25 g/100 kg seed, 3-5 kg/ha in seed furrow), rice (150-250 g/ha foliar), cotton (45-75 g/100 kg seed), sugar beet (500 g/ha foliar), vegetables (1250-1500 g/ha drench and incorporation in field at transplanting), ornamentals and turf. In particular, control of black scurf of potatoes, sheath blight of rice, and damping-off of ornamentals. Formulation types DP; DS; FS; SC; WP. Compatibility Compatible with a number of other fungicides and insecticides, e.g. edifenphos, captan, thiram, fenthion. Selected products: 'Monceren' (Bayer CropScience); 'Vicuron' (Vipesco); mixtures: 'Gaucho M' (+ thiram+ imidacloprid) (Bayer CropScience); 'Monceren G' (+ imidacloprid) (Germany) (Bayer CropScience); 'Monceren IM' (+ imazalil sulfate) (UK, Ireland) (Bayer CropScience); 'Prestige' (+ imidacloprid) (Bayer CropScience)
OTHER PRODUCTS
'Penny' (Me2); 'Trotis' (Bayer CropScience) mixtures: 'Monceren Admire' (+ imidacloprid) (Bayer CropScience); 'Monceren Star' (+ imidacloprid) (Bayer CropScience); 'Monceren T' (+ thiram) (Bayer CropScience); 'Teevic' (+ thiram) (Bayer CropScience); 'Turfsiba' (+ tebuconazole) (spray, Japan) (Bayer CropScience)
ANALYSIS
Product analysis by lc (CIPAC Handbook, 1992, E, 173-8). Residues determined by glc; details available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 (24 h) for rats and mice >2000 mg/kg. Non-irritating to skin and eyes (rabbits). Not a skin sensitiser. Inhalation LC50 (4 h) for rats >268 mg/m3 air (aerosol); >5130 mg/m3 air (dust). NOEL (2 y) for male rats 50, female rats 500, dogs 100, male and female mice 500 mg/kg diet. ADI 0.02 mg/kg b.w. Other Non-teratogenic, non-carcinogenic, non-mutagenic. Toxicity class WHO (a.i.) U; EPA (formulation) IV
ECOTOXICOLOGY
Birds LD50 for bobwhite quail >2000 mg/kg. Fish LC50 (96 h) for rainbow trout >690 mg/l (11 °C), bluegill sunfish 127 mg/l (19 °C). Daphnia EC50 (48 h) 0.27 mg/l. Algae EC50 (96 h) for Scenedesmus 0.56 mg/l. Bees Not hazardous to bees; LD50 (oral and contact) >100 mg/bee. Worms LC50 (14 d) >1000 mg/kg dry soil. Other beneficial spp. Not toxic for carabid beetles (Calathus fuscipes, Pterostichus melanarius) up to 25 kg/ha. Under practical conditions, no negative effects on soil micro-organisms.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, up to 74% is eliminated within 3 days in the urine and faeces, either as the unchanged material or as metabolites. Eleven metabolites have been identified (I. Ueyama et al., J. Agric. Food Chem., 1982, 30(6), 1061-1067). The main metabolic pathway comprises hydroxylation of the phenyl group to various diol and triol compounds which are frequently eliminated as sulfate and glucuronic acid conjugates. Plants Degradation only to a low degree. The hydroxylated metabolites (partly as conjugates) can be considered to be the main metabolites. Soil/Environment Leaching and adsorption behaviour in soil characterises the compound as slightly mobile. Degraded in soil very effectively in laboratory studies. Based on determined DT50 values, the compound can be classified as moderately stable to stable. The main metabolites are derivatives of p-chlorobenzylamine and p-chlorobenzylformamide.
|