|
oxasulfuron
Herbicide
HRAC B WSSA 2; sulfonylurea
NOMENCLATURE
Common name oxasulfuron (BSI, pa ISO)
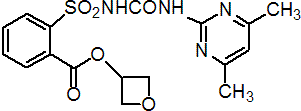
IUPAC name oxetan-3-yl 2-[(4,6-dimethylpyrimidin-2-yl)-carbamoylsulfamoyl]benzoate
Chemical Abstracts name 3-oxetanyl 2-[[[[(4,6-dimethyl-2-pyrimidinyl)amino]carbonyl]amino]sulfonyl]benzoate
CAS RN [144651-06-9] Development codes CGA-277476 (Ciba-Geigy)
PHYSICAL CHEMISTRY
Mol. wt. 406.4 M.f. C17H18N4O6S Form White, odourless powder. M.p. 158 °C (decomp.) V.p. <2 ´ 10-3 mPa (25 °C) KOW logP = 0.75 (pH 5), -0.81 (pH 7), -2.2 (pH 8.9) Henry <3.2 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.41 Solubility In water 52 ppm (pH 5.1, 25 °C); in buffer, 63 (pH 5.0), 1700 (pH 6.8), 19 000 (pH 7.8) mg/l (15 °C). In methanol 1500, acetone 9300, toluene 320, n-octanol 99, n-hexane 2.2, ethyl acetate 2300, dichloromethane 6900 (all in mg/l, 25 °C). pKa 5.1
COMMERCIALISATION
History Reported by R. L. Brooks et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1995, 1, 79). Introduced by Novartis Crop Protection AG (now Syngenta AG) in 1996. Patents US 5209771; EP 0496701 Manufacturers Syngenta
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity derives from rapid metabolism in the crop. Metabolic basis of selectivity in sulfonylureas reviewed (M. K. Koeppe & H. M. Brown, Agro-Food-Industry, 6, 9-14 (1995)). Mode of action Readily taken up by shoots and roots and translocated to meristematic tissues. Leaves turn yellow or red, followed by death after 1 to 3 weeks. Uses Under development for broad-leaved and grass weed control in soya beans, at 60-90 g/ha. Formulation types WG. Selected products: 'Chart' (Syngenta); 'Dynam' (Syngenta); 'Expert' (Syngenta)
OTHER PRODUCTS
'Rebit GT' (Syngenta)
ANALYSIS
Methods for sulfonylurea residues in crops, soil and water reviewed (A. C. Barefoot et al., Proc. Br. Crop Prot. Conf. - Weeds, 1995, 2, 707).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. No skin or eye irritation (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 for rats >5.08 mg/l air. NOEL (2 y) for rats 8.3 mg/kg b.w. daily; (18 mo) for mice 1.5 mg/kg b.w. daily; (1 y) for dogs 1.3 mg/kg b.w. daily. ADI 0.013 mg/kg b.w. Other Non-mutagenic. Toxicity class WHO (a.i.) U (company classification)
ECOTOXICOLOGY
Birds Acute oral LD50 for quail and mallard ducks >2250 mg/kg. Dietary LC50 for quail and mallard ducks >5620 ppm. Fish LC50 for bluegill sunfish >111, trout >116 mg/l. Daphnia EC50 (48 h) >89.4 mg/l. Algae EC50 for Selenastrum capricornutum 0.145, Navicula >20 mg/l. Bees LD50 >25 mg/bee. Worms LC50 for earthworms >1000 mg/kg.
ENVIRONMENTAL FATE
Animals The majority (70-80%) of the applied dose is excreted in urine with no accumulation in tissues; depletion t½ c. 7-14 h. The metabolic route involves hydroxylation of the pyrimidine methyl, hydrolysis of the oxetane ring and cleavage of the sulfonylurea bridge. Plants The major metabolite is saccharine (0.002 ppm in mature beans); small amounts of oxetane alcohol are also formed. Metabolism follows a similar route to animals. Soil/Environment Soil DT50 (lab.) 10 d, (field) <2 w. Breakdown is primarily microbial and hydrolytic; independent of soil pH, organic matter content or soil structure. Koc 44 (13 soils).
|