|
metsulfuron-methyl
Herbicide
HRAC B WSSA 2; sulfonylurea
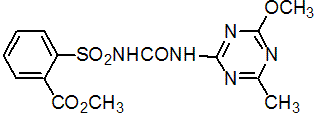
NOMENCLATURE
metsulfuron-methyl
Common name metsulfuron-methyl
IUPAC name methyl 2-(4-methoxy-6-methyl-1,3,5-triazin-2-ylcarbamoylsulfamoyl)benzoate
Chemical Abstracts name methyl 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]amino]sulfonyl]benzoate
CAS RN [74223-64-6] Development codes DPX-T6376 (DuPont); IN-T6376
metsulfuron
Common name metsulfuron (BSI, WSSA, ANSI, draft E-ISO, (m) draft F-ISO)
IUPAC name 2-(4-methoxy-6-methyl-1,3,5-triazin-2-ylcarbamoylsulfamoyl)benzoic acid
Chemical Abstracts name 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]amino]sulfonyl]benzoic acid
CAS RN [79510-48-8]
PHYSICAL CHEMISTRY
metsulfuron-methyl
Composition Tech. is >96%. Mol. wt. 381.4 M.f. C14H15N5O6S Form Colourless crystals; (tech., off-white solid, with a faint ester-like odour). M.p. 162 °C V.p. 3.3 ´ 10-7 mPa (25 ºC) KOW logP = 0.018 (pH 7, 25 °C) Henry 4.5 ´ 10-11 Pa m3 mol-1 (pH 7, 25 ºC) S.g./density 1.447 (20 °C) Solubility In water 0.548 (pH 5), 2.79 (pH 7), 213 (pH 9) (all in g/l, 25 ºC). In hexane 5.84 ´ 10-1, ethyl acetate 1.11 ´ 104, methanol 7.63 ´ 103, acetone 3.7 ´ 104, dichloromethane 1.32 ´ 105, toluene 1.24 ´ 103 (all in mg/l, 25 ºC). Stability Photolytically stable. Hydrolysis DT50 (25 °C), 22 d (pH 5), stable (pH 7 and 9). pKa 3.8 (20 °C)
metsulfuron
Mol. wt. 367.3 M.f. C13H13N5O6S Form Solid.
COMMERCIALISATION
History Herbicidal properties of metsulfuron-methyl reported by R. I. Doig et al. (Proc. Int. Congr. Plant Prot., 10th, 1983, 1, 324). Introduced by E. I. du Pont de Nemours and Co.; first approval in 1984. Patents US 4370480 Manufacturers DuPont; IPESA; JIE; Reposo; Sannong; Sharda; Sundat; Tide
APPLICATIONS
metsulfuron-methyl
Biochemistry Affects sensitive weeds through inhibition of the enzyme acetolactate synthase (ALS). Inhibition of ALS leads to the rapid cessation of cell division and subsequent growth processes in plants. Mode of action Selective systemic herbicide absorbed through the roots and foliage and translocated to the apex of the plants. Symptoms appear within days with death within 2-4 weeks. Uses Controls a wide range of grass and broad-leaved weeds in wheat, barley, rice, oats and triticale by post-emergence application, at 4-8 g/ha. Formulation types WG. Selected products: 'Alli? (DuPont); 'Ally' (DuPont); 'Escort' (DuPont); 'Gropper' (DuPont); 'Flumen' (Barclay); 'Nicanor' (Makhteshim-Agan); 'Quit' (Sanonda); 'Retador' (Reposo); 'Rosulfuron' (Rotam); 'Timefron' (Tide); mixtures: 'Canvas' (+ thifensulfuron-methyl+ tribenuron-methyl) (DuPont)
OTHER PRODUCTS
metsulfuron-methyl
'Gaio' (DuPont); 'Gem 690' (DuPont); 'Jubilee' (DuPont); 'Lorate' (DuPont); 'Simba' (DuPont); 'Luger' (Chemiplant); 'Malban' (IPESA, Makhteshim-Agan); 'Metgard' (Makhteshim-Agan); 'Pilarcort' (Pilarquim); 'Purestand' (Nufarm Americas); 'Rozar' (Makhteshim-Agan); 'Triticas' (CAS); 'Valuron' (Makhteshim-Agan) mixtures: 'Alli?Express' (+ carfentrazone-ethyl) (DuPont); 'Ally Express' (+ carfentrazone-ethyl) (DuPont); 'Almix' (+ chlorimuron-ethyl) (DuPont); 'Finesse' (+ chlorsulfuron) (DuPont); 'Harmony M' (+ thifensulfuron-methyl) (DuPont); 'Lexus XPE' (+ flupyrsulfuron-methyl-sodium) (DuPont); 'Scoop' (+ thifensulfuron-methyl) (DuPont); 'Sindax' (+ bensulfuron-methyl) (DuPont); 'Spéléo' (+ flupyrsulfuron-methyl-sodium) (DuPont); 'Neptune' (+ mecoprop-P) (Headland); 'Pasture MD' (+ 2,4-D+ dicamba) (Nufarm Americas); 'Sulfonil' (+ propanil) (Crystal) Discontinued products: 'Alrip' * (DuPont); 'PartiSan' * (Sanonda) mixtures: 'DP 911' * (+ tribenuron-methyl) (DuPont); 'DP 928' * (+ thifensulfuron-methyl) (DuPont)
ANALYSIS
Product and residue analysis by hplc/ms/ms (Handbook of Residue Analytical Methods, pages 400-410; CIPAC Handbook, 1998, H, 204)
MAMMALIAN TOXICOLOGY
metsulfuron-methyl
Oral Acute oral LD50 for male and female rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Not a skin or eye irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for male and female rats >5 mg/l air. NOEL (18 mo) for mice 5000 ppm; (2 y) for rats 500 ppm; (1 y) for male dogs 500 ppm; (1 y) for female dogs 5000 ppm. ADI 0.22 mg/kg Other Not genotoxic; non-teratogenic. Toxicity class WHO (a.i.) U; EPA (formulation) IV EC classification N; R50, R53
ECOTOXICOLOGY
metsulfuron-methyl
Birds Acute oral LD50 for mallard ducks >2510 mg/kg. Dietary LC50 (8 d) for mallard ducks and bobwhite quail >5620 mg/kg diet. Fish LC50 (96 h) for rainbow trout and bluegill sunfish >150 mg/l. Daphnia EC50 (48 h) >120 mg/l. Algae EC50 (72 h) for green algae 0.157 mg/l. Other aquatic spp. EC50 for Lemna gibba 0.36 mg/l. Bees Non-toxic to bees; LD50 (oral) >44.3 mg/bee; (contact) >50 mg/bee. Worms LC50 >1000 mg/kg.
ENVIRONMENTAL FATE
Animals In mammals, following oral administration, metsulfuron-methyl is excreted predominantly unchanged. The methoxycarbonyl and sulfonylurea groups are only partly degraded, by O-demethylation and hydroxylation. Plants In plants, undergoes complete degradation within a few days, by hydrolysis and conjugation. In addition to the hydroxymethyl analogue, other metabolites identified include methyl 2-(aminosulfonyl)benzoate and 2-(aminosulfonyl)benzoic acid. Rapidly metabolised within cereal plants. Soil/Environment In soil, metsulfuron-methyl is broken down both by chemical hydrolysis and by microbial degradation. DT50 (ave.) in a range of field soils is 52 days, with faster degradation in acidic soils.
|