|
RU 15525
Insecticide
IRAC 3; pyrethroid
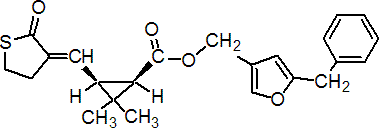
NOMENCLATURE
IUPAC name 5-benzyl-3-furylmethyl (E)-(1R,3S)-2,2-dimethyl-3-(2-oxothiolan-3-ylidenemethyl)cyclopropanecarboxylate
Roth: 5-benzyl-3-furylmethyl (E)-(1R)-cis-2,2-dimethyl-3-(2-oxothiolan-3-ylidenemethyl)cyclopropanecarboxylate
Chemical Abstracts name [1R-[1a,3a(E)]]-[5-(phenylmethyl)-3-furanyl]methyl 3-[(dihydro-2-oxo-3(2H)-thienylidene)methyl]-2,2-dimethylcyclopropanecarboxylate
Other names kadethrin; kadethrine CAS RN [58769-20-3] EEC no. 261-433-0 Development codes RU 15 525 (Roussel Uclaf) Official codes ENT 29 117; AI3-29 117
PHYSICAL CHEMISTRY
Composition Tech. grade is ³93% pure m/m of stated stereoisomer. Mol. wt. 396.5 M.f. C23H24O4S Form Yellow-brown, viscous oil. M.p. 31 ºC V.p. <0.1 mPa (20 ºC) Solubility Practically insoluble in water. Soluble in dichloromethane, ethanol (10 g/kg), benzene, toluene, xylene, acetone, piperonyl butoxide. Slightly soluble in kerosene. Stability Hydrolysed by aqueous alkalis. Rapidly decomposed by light (more slowly in mineral oils). Unstable to heat. Specific rotation [a]D20 +16?to +19?(c. 50 g tech./l toluene) F.p. >100 ºC
COMMERCIALISATION
History Insecticide reported by J. Martel & J. Buendia (Int. Congr. Pestic. Chem. IUPAC, 3rd, 1974) and by J. Lhoste & F. Rauch (Pestic. Sci., 1976, 7, 247). Introduced by Roussel Uclaf (now Bayer CropScience). Patents FR 2097244; US 3842177
APPLICATIONS
Biochemistry Acts on the nervous system of insects, disturbs the function of neurons by interaction with the sodium channel. Mode of action Insecticide with contact action. Gives rapid knockdown. Uses Control of household insect pests, particularly houseflies, mosquitoes, and cockroaches. Normally used in combination with other pyrethroid insecticides and synergists. Formulation types Aerosol; OL; TC. Compatibility Incompatible with alkaline materials.
OTHER PRODUCTS
'Kadethrin' (Bayer CropScience); 'Kadethrine' (Bayer CropScience)
ANALYSIS
Product analysis by glc or hplc; details available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 1324, female rats 650, dogs >1000 mg/kg. Skin and eye Acute percutaneous LD50 for female rats >3200 mg/kg. Slight irritant to skin, eyes, and respiratory tract. Inhalation Inhalation trials (10 d) with rats and guinea pigs at 200 times normal dose in aerosols tolerated perfectly. NOEL (90 d) for rats 25, dogs 15 mg/kg b.w. daily. Other No teratogenic effect was observed in mice, rats or rabbits.
ECOTOXICOLOGY
Fish Toxic to fish; LC50 (96 h) for rainbow trout 0.13 mg/l. Bees Toxic to bees.
ENVIRONMENTAL FATE
Animals In rats, following oral administration, there is rapid metabolism (by opening of the thiolactone ring, cleavage of the ester, and ring hydroxylation), and rapid excretion. See K. Ohsawa & J. E. Casida (J. Agric. Food Chem., 1980, 28, 250-255).
|