|
isoxaflutole
Herbicide
HRAC F2 WSSA 28; isoxazole
NOMENCLATURE
Common name isoxaflutole (BSI, pa ISO)
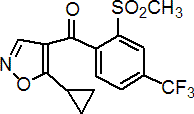
IUPAC name 5-cyclopropyl-1,2-oxazol-4-yl a,a,a-trifluoro-2-mesyl-p-tolyl ketone
Chemical Abstracts name (5-cyclopropyl-4-isoxazolyl)[2-(methylsulfonyl)-4-(trifluoromethyl)phenyl]methanone
CAS RN [141112-29-0] Development codes RPA 201772 (Rhône-Poulenc)
PHYSICAL CHEMISTRY
Composition Tech. is c. 98% pure. Mol. wt. 359.3 M.f. C15H12F3NO4S Form Off-white or pale yellow solid. M.p. 140 °C V.p. 1 ´ 10-3 mPa (25 °C) KOW logP = 2.32 Henry 1.87 ´ 10-5 Pa m3 mol-1 (20 °C) S.g./density 1.590 Solubility In water 6.2 mg/l (pH 5.5, 20 °C). Stability Stable to heat (14 d at 54 °C) and to light. DT50 in water 1 d at pH 7.
COMMERCIALISATION
History Under development from 1992. Reported by B. M. Luscombe et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1995, 1, 35). Patents EP 0527036 Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Rapidly metabolised in plants, and in the soil, by opening of the isoxazole ring, to form a diketonitrile, which is the active species (K. E. Pallett et al., Pest Manag. Sci. 57, 133 (2001) and refs. therein); this is a p-hydroxyphenyl pyruvate dioxygenase inhibitor. This enzyme converts p-hydroxyphenyl pyruvate to homogentisate, a key step in plastoquinone biosynthesis. Inhibition leads to indirect inhibition of carotenoid biosynthesis, giving rise to chlorosis of new growth. Mode of action Systemic by either root or foliar uptake. Uses Broad-spectrum grass and broad-leaved weed control in maize and sugar cane. Applied at 75-140 g/ha pre-emergence or pre-plant; the spectrum can be enhanced by mixture with other active ingredients. Formulation types SC; WG; WP. Selected products: 'Balance' (Bayer CropScience); 'Merlin' (Bayer CropScience, Certis Europe); mixtures: 'Epic' (+ flufenacet) (Bayer CropScience)
OTHER PRODUCTS
'Alliance' (Bayer CropScience); 'Converge' (Bayer CropScience); 'Provence' (Bayer CropScience) mixtures: 'Acajou' (+ aclonifen) (Bayer CropScience); 'Atoll' (+ atrazine) (Bayer CropScience); 'Cadou Star' (+ flufenacet) (Bayer CropScience); 'Lagon' (+ aclonifen) (Bayer CropScience)
ANALYSIS
By hplc with u.v. detection.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Not a skin irritant; minimal eye irritation (rabbits). Not a skin sensitiser. Inhalation LC50 (4 h) for rats >5.23 mg/l. NOEL (2 y) for rats 2 mg/kg daily. Other Non-mutagenic, non-neurotoxic. Toxicity class EPA (formulation) III EC classification R63| N; R50, R53
ECOTOXICOLOGY
Birds Acute oral LD50 (14 d) for quail and mallard ducks >2150 mg/kg; dietary LC50(8 d) >5000 ppm. Fish Non-toxic at limit of water solubility. Daphnia Non-toxic at limit of water solubility. Algae EC50 for Selenastrum capricornutum 0.016 mg/l. Other aquatic spp. EC50 (96 h) for Eastern oyster (Crassostrea virginica) 3.4 mg/l, mysid shrimp (Mysidopsis bahia) 18 mg/l. Bees LD50 (oral and contact) >100 mg/bee. Worms Non-toxic at 1000 mg/kg.
ENVIRONMENTAL FATE
Animals Following oral administration, isoxaflutole is rapidly excreted. Plants Plant metabolism study demonstrated that residue levels at harvest are very low, and comprise mainly a non-toxic metabolite. Soil/Environment In laboratory soil studies, degradation proceeds via hydrolysis and microbial degradation, with final mineralisation to CO2. Isoxaflutole and its major metabolites are potentially mobile in soil under simulated high rainfall; however field studies indicate that residues remain in the surface horizons; after 4 months, virtually no residues remain in the soil.
|