|
iprovalicarb
Fungicide
FRAC U1, H2; amino acid amide carbamate
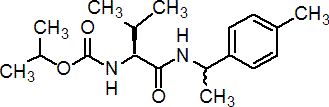
NOMENCLATURE
Common name iprovalicarb (BSI, pa ISO)
IUPAC name isopropyl 2-methyl-1-[(1-p-tolylethyl)carbamoyl]-(S)-propylcarbamate
Chemical Abstracts name 1-methylethyl [(1S)-2-methyl-1-[[[1-(4-methylphenyl)ethyl] amino]carbonyl]propyl]carbamate
CAS RN [140923-17-7]; [140923-25-7] (SR)- isomer Development codes SZX 0722; SZX 722 (both Bayer)
PHYSICAL CHEMISTRY
Composition A mixture of (S,S)- and (S,R)- diastereoisomers. Mol. wt. 320.4 M.f. C18H28N2O3 Form White to yellow powder. M.p. 183 °C (SR)-; 199 °C (SS)-; 163-165 °C (mixture) V.p. 4.4 ´ 10-5 (SR)-; 3.5 ´ 10-5 (SS)-; 7.7 ´ 10-5 (mixture) (all mPa, 20 °C) KOW logP = 3.2 ((SR)- and (SS)- diastereoisomers) Henry 1.3 ´ 10-6 (SR)-; 1.6 ´ 10-6 (SS)- (both Pa m3 mol-1, 20 °C, calc.) S.g./density 1.11 (20 °C) Solubility In water 11.0 ((SR)- diastereoisomer), 6.8 ((SS)- diastereoisomer) (both mg/l, 20 °C). In dichloromethane 97 (SR)-, 35 (SS)-; toluene 2.9 (SR)-, 2.4 (SS)-; acetone 22 (SR)-, 19 (SS)-; n-hexane 0.06 (SR)-, 0.04 (SS)-; isopropanol 15 (SR)-, 13 (SS)- (all in g/l, 20°C).
COMMERCIALISATION
History Discovered in 1989. Reported by K. Stenzel et al. (Proc. Br. Crop Prot. Conf. - Pests Dis., 1998, 2, 367). Introduced by Bayer AG in 1999. Patents DE 04026966 Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Affects growth of germ tubes of zoospores and sporangia, growth of mycelium and sporulation of Oomycete fungi. Mode of action Protective, curative and eradicative, systemic fungicide, distributed in the transpiration stream. Uses Under development for protectant control of Oomycete fungi, such as Plasmopara viticola in grapes, at 120-150 g/ha, Phytophthora infestans on potatoes and tomatoes, Pseudoperonospora cubensis on cucumber, and Peronospora tabacina on tobacco, all at 180-220 g/ha. Used only in combination products. Formulation types SC; WG; WP. Selected products: 'Melody' (various mixtures) (Bayer CropScience)
OTHER PRODUCTS
Mixtures: 'Invento' (+ propineb) (Bayer CropScience); 'Mandore' (+ mancozeb) (France) (Bayer CropScience); 'Melody Combi' (+ propineb+ folpet) (Bayer CropScience); 'Melody Compact' (+ copper oxychloride) (Bayer CropScience); 'Melody Duo' (+ propineb) (Bayer CropScience); 'Melody Med' (+ mancozeb) (Bayer CropScience); 'Melody Multi' (+ tolylfluanid) (Germany) (Bayer CropScience); 'Melody Star' (+ azoxystrobin) (Germany, Italy, Portugal) (Bayer CropScience); 'Ocarina' (+ copper oxychloride) (Bayer CropScience); 'Odena' (+ folpet) (France) (Bayer CropScience); 'Positron Duo' (+ propineb) (Latin America) (Bayer CropScience); 'Sirbel' (+ folpet) (France) (Bayer CropScience); 'Yorel' (+ mancozeb) (France) (Bayer CropScience)
ANALYSIS
Details of methods for product and residue analysis available from Bayer CropScience.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg. Non-irritating to skin and eyes (rabbits); not a skin sensitiser (guinea pigs). Inhalation LC50 for rats >4977 mg/m3 air (dust). NOEL (2 y) for female rats 500, male rats 5000, male mice 1400, female mice 7000 mg/kg diet; (1 y) for dogs <80 mg/kg diet. ADI 0.015 mg/kg b.w. (EU proposed). Other Non-neurotoxic, non-mutagenic, non-embryotoxic, non-carcinogenic. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5000 mg/kg feed. Fish LC50 (96 h) for rainbow trout >22.7, bluegill sunfish >20.7 mg/l. Daphnia EC50 (48 h) >19.8 mg/l. Algae Eb/rC50 (72 h) for green algae (Selenastrum capricornutum) >10.0 mg/l. Bees LD50 (48 h) for honeybees (oral) >199 mg/bee; (contact) >200 mg/bee. Worms LC50 (14 d) for Eisenia foetida >1000 mg/kg dry soil. Other beneficial spp. No negative effects on Typhlodromus pyri at 460 g/ha, on Poecilus cupreus at 2 ´ 461 g/ha, on Coccinella septempunctata at 550 g/ha, or on Aphidius rhopalosiphi at 450 g/ha.
ENVIRONMENTAL FATE
Animals After oral administration of radiolabelled iprovalicarb to rats and to lactating goats, the radioactivity was readily excreted via faeces and urine. Iprovalicarb was metabolised extensively; the major components of the residue were the carboxylic acid derivative, formed by oxidation of the ring methyl, and the active substance itself. Plants When the above-ground parts of treated grape, tomato and potato plants were sampled, the major portion of the residues was found on the surface. The rate of degradation in plants was quite low, and iprovalicarb itself was always the major component of the residue. Soil/Environment Iprovalicarb was thoroughly degraded in soil under aerobic conditions, ultimately forming CO2; DT50 4-8 weeks. On the basis of its Koc values, iprovalicarb can be classified as being in the range from "a mobile substance" to "a substance of low mobility".
|