|
iodosulfuron-methyl-sodium
Herbicide
HRAC B WSSA 2; sulfonylurea
NOMENCLATURE
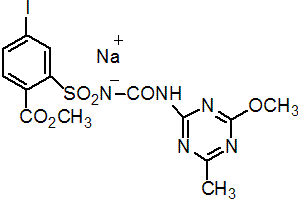
Common name iodosulfuron-methyl-sodium (BSI, pa ISO)
IUPAC name methyl 4-iodo-2-[3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)ureidosulfonyl]benzoate, sodium salt
Chemical Abstracts name methyl 4-iodo-2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]amino]sulfonyl]benzoate, sodium salt
CAS RN [144550-36-7] ; [185119-76-0] acid Development codes AE F115008 (AgrEvo); AE F145740 (acid)
PHYSICAL CHEMISTRY
Composition The species used in practice is the sodium salt of the methyl ester. Where data are stated to refer to the 'acid', they apply to the diacid (carboxylic acid and sulfonyl urea). Mol. wt. 529.2; 493.2 (acid) M.f. C14H13IN5NaO6S; C13H12IN5O6S (acid) Form Almost colourless/light beige, crystalline powder. M.p. 152 °C V.p. 6.7 ´ 10-6 mPa (25 °C) KOW logP = 1.07 (pH 5), -0.70 (pH 7), -1.22 (pH 9). Solubility In water 0.16 (pH 5), 25 (pH 7), 65 (pH 9) (all in g/l, 20 °C).
COMMERCIALISATION
History Reported by E. Hacker et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 15). Under development by AgrEvo GmbH (now Bayer CropScience). Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Selectivity to cereals is due to differential degradation, compared with that in grass weeds, which is enhanced by addition of the safener mefenpyr-diethyl (q.v.) Uses For post-emergence control of grass and broad-leaved weeds in winter, spring and durum wheat, triticale and rye, applied at 10 g/ha, in mixture with the safener mefenpyr-diethyl (q.v.). Formulation types WG; Oil formulation. Selected products: 'Hussar' (Bayer CropScience)
OTHER PRODUCTS
'Chekker' (Bayer CropScience); 'Husar' (Germany) (Bayer CropScience); 'Sekator' (Bayer CropScience) mixtures: 'Archipel' (+ mefenpyr-diethyl+ mesosulfuron-methyl) (Bayer CropScience); 'Atlantis' (+ mefenpyr-diethyl+ mesosulfuron-methyl) (Bayer CropScience); 'Cossack' (+ mefenpyr-diethyl+ mesosulfuron-methyl) (Bayer CropScience); 'Fortuna' (+ isoxadifen-ethyl+ foramsulfuron) (Bayer CropScience); 'Hoestar Super' (+ amidosulfuron) (Bayer CropScience); 'MaisTer' (+ isoxadifen-ethyl+ foramsulfuron) (Bayer CropScience); 'Tribute' (+ foramsulfuron) (Bayer CropScience)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats 2678 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg. Non-irritating to skin and eyes (rabbits); not a skin sensitiser (guinea pigs). Other Non-mutagenic in vitro and in vivo.
ECOTOXICOLOGY
Fish LC50 for fish 100 mg/l. Algae EC50 for green algae 0.02 mg/l. Other aquatic spp. EC50 for Lemna gibba 0.8 mg/l.
ENVIRONMENTAL FATE
Soil/Environment Photodegradation DT50 c. 50 d. Abiotic hydrolysis DT50 31 d (pH 5), >365 d (pH 7), 362 d (pH 9) (20 °C). Soil DT50 1-5 d (7-10 d with low soil moisture); degradation is mainly microbial. Iodosulfuron-methyl and its metabolites have almost no vertical movement in soil; lysimeter and computer simulation studies indicate that neither iodosulfuron-methyl nor its metabolites would be transported to soil layers deeper than 1 m.
|