|
flusulfamide
Fungicide
FRAC 36, U6; benzenesulfonamide
NOMENCLATURE
Common name flusulfamide (BSI, draft E-ISO)
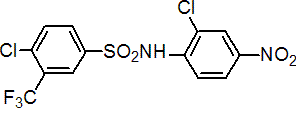
IUPAC name 2',4-dichloro-a,a,a-trifluoro-4'-nitro-m-toluenesulfonanilide
Chemical Abstracts name 4-chloro-N-(2-chloro-4-nitrophenyl)-3-(trifluoromethyl)benzenesulfonamide
CAS RN [106917-52-6] Development codes MTF-651 (Mitsui)
PHYSICAL CHEMISTRY
Mol. wt. 415.2 M.f. C13H7Cl2F3N2O4S Form Light yellow crystals. M.p. 169.7-171.0 °C V.p. 9.9 ´ 10-7 mPa (40 °C) KOW logP = 2.8?.5 S.g./density 1.739 g/cm3 (23 °C) Solubility In water 2.9 mg/kg (25 ºC). In methanol 24, acetone 314, xylene 14, tetrahydrofuran 592 (all in g/kg, 25 ºC). Stability Stable between 35 ºC and 80 ºC in the dark for 90 d. Stable in acidic and in alkaline media.
COMMERCIALISATION
History Fungicide discovered in 1986 by Mitsui Toatsu Chemicals, Inc. (now Mitsui Chemicals Inc.). Patents EP 199433 Manufacturers Mitsui
APPLICATIONS
Mode of action Inhibits spore germination. Uses Used as a soil treatment for control of Plasmodiophora brassicae in brassicas and Polymyxa betae (fungal vector of beet yellow vein virus causing rhizomania disease) in sugar beet, at 0.6-0.9 kg/ha. Also controls Pythium, Rhizoctonia, Phytophthora, and Fusarium spp. Formulation types DP; SC. Selected products: 'Nebijin' (Mitsui)
ANALYSIS
Product analysis by hplc.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for male rats 180, female rats 132 mg/kg. Skin and eye Acute percutaneous LD50 for male and female rats >2000 mg/kg. Slight eye irritant; not a skin irritant (rabbits). Not a skin sensitiser. Inhalation LC50 (4 h) for male and female rats 0.47 mg/l.
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail 66 mg/kg. Fish TLm (96 h) for bluegill sunfish 1.2 mg/l. Daphnia EC50 (48 h) 0.29 mg/l. Algae ErC50 (72 h) 4.2 mg/l. Bees LD50 >200 mg/bee. Worms >9 kg/ha.
ENVIRONMENTAL FATE
Animals In rats, the parent compound, 4-chloro-N-(2-chloro-4-hydroxyphenyl)-a,a,a-trifluoro-m-toluenesulfonamide and 4-chloro-a,a,a-trifluoro-m-toluenesulfonamide are found. Plants In cabbage, the parent compound, 4-chloro-N-(2-chloro-4-formylaminophenyl)-a,a,a-trifluoro-m-toluenesulfonamide, 4-chloro-a,a,a-trifluoro-m-toluenesulfonamide and 4-chloro-a,a,a-trifluoro-m-toluenesulfonate are found.
|