|
flupyrsulfuron-methyl-sodium
Herbicide
HRAC B WSSA 2; sulfonylurea
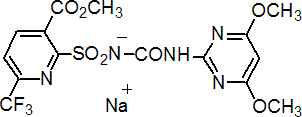
NOMENCLATURE
Common name flupyrsulfuron-methyl-sodium (BSI, pa ISO, ANSI)
IUPAC name methyl 2-(4,6-dimethoxypyrimidin-2-ylcarbamoylsulfamoyl)-6-trifluoromethylnicotinate monosodium salt
Chemical Abstracts name methyl 2-[[[[(4,6-dimethoxy-2-pyrimidinyl)amino]carbonyl]amino]sulfonyl]-6-(trifluoromethyl)-3-pyridinecarboxylate monosodium salt
CAS RN [144740-54-5]; acid [150315-10-9] Development codes DPX-KE459 (DuPont); DPX-JE138 (DuPont); IN-KE459
PHYSICAL CHEMISTRY
Composition Tech. is >90.3 g/kg. Mol. wt. 487.3 M.f. C15H13F3N5NaO7S; (acid, C14H12F3N5O7S) Form White powder, with a pungent, astringent odour. M.p. 165-170 °C V.p. 1 ´ 10-6 mPa (20 °C) KOW logP = 0.96 (pH 5), 0.10 (pH 6) (both 20 °C) Henry 1 ´ 10-8 Pa m3 mol-1 (pH5, 20 °C) (calc.) S.g./density 1.55 (19.3 °C) Solubility In water 62.7 (pH 5), 603 (pH 6) (both mg/l, 20 °C). In dichloromethane 0.60, acetone 3.1, ethyl acetate 0.49, acetonitrile 4.3, hexane <0.001, methanol 5.0 (all in g/l, 20 °C). Stability DT50 44 d (pH 5), 12 d (pH 7), 0.42 d (pH 9) (20 °C). Stable in most organic solvents. pKa 4.9
COMMERCIALISATION
History Reported by S. R. Teaney et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1995, 1, 49). Manufacturers DuPont
APPLICATIONS
Biochemistry Affects sensitive weeds through inhibition of the enzyme acetolactate synthase (ALS). Inhibition of ALS leads to the rapid cessation of cell division and subsequent growth processes in plants. Mode of action Post-emergence, selective herbicide acting primarily through foliar uptake with little or no soil activity Uses Selective herbicide, which controls grass (primarily) blackgrass (Alopecurus myosuroides) and broad-leaf weeds in cereals. Applied post-emergence at 10 g/ha. Formulation types WG. Selected products: 'Lexus' (DuPont)
OTHER PRODUCTS
'Balance' (DuPont); 'Ductis' (DuPont); 'Lexus Solo' (DuPont); 'Oklar' (DuPont) mixtures: 'Lexus Class' (+ carfentrazone-ethyl) (DuPont); 'Lexus Millenium' (+ thifensulfuron-methyl) (DuPont); 'Lexus XPE' (+ metsulfuron-methyl) (DuPont); 'Millenium' (+ thifensulfuron-methyl) (DuPont); 'Spéléo' (+ metsulfuron-methyl) (DuPont)
ANALYSIS
Product and residue analysis by hplc/ms/ms (Handbook of Residue Analytical Methods, pp 400-410).
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Not a skin or eye irritant (rabbits); not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.8 mg/l. NOEL (90 d) for male rats 124 mg/kg daily; (2 y) for male rats 14.2 mg/kg daily; (1 y) for male dogs 146.3 mg/kg daily. ADI 0.035 mg/kg. Other Non-mutagenic (Ames test), not genotoxic. Toxicity class WHO (a.i.) U EC classification N; R50, R53
ECOTOXICOLOGY
Birds Oral LD50 for mallard ducks >2250 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5620 ppm. Fish LC50 (96 h) for carp 820, rainbow trout 470 mg/l. Daphnia LC50 (48 h) 721 mg/l. Algae EC50 (120 h) for green algae 0.004 mg/l. Other aquatic spp. EC50 (14 d) for Lemna gibba 0.003 mg/l. Bees LD50 (contact) >25 mg/bee; (dietary) >30 mg/bee. Worms LC50 >1000 mg/kg.
ENVIRONMENTAL FATE
Low Kow suggests that bioaccumulation should not occur. Animals In rats, rapid adsorption, metabolism and elimination of the administered dose occurred, with 90% excreted in the faeces and urine within 96 hours. Plants Flupyrsulfuron-methyl is rapidly metabolised in plants, by nucleophilic substitution of the sulfonyl group by gluthathione or by the intramolecular ipso nitrogen on the sulfonylurea bridge. Soil/Environment Lab. DT50 (ave.) in aerobic soils is 14 d. Under field conditions, ave. DT50 and DT90 are 14 and 47 d. Degradation is faster in alkaline soils; in acid soils, hydrolysis of the sulfonylurea bridge occurs.
|