|
flumetsulam
Herbicide
HRAC B WSSA 2; triazolopyrimidine
NOMENCLATURE
Common name flumetsulam (BSI, pa E-ISO, ANSI)
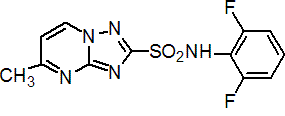
IUPAC name 2',6'-difluoro-5-methyl[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonanilide
Chemical Abstracts name N-(2,6-difluorophenyl)-5-methyl[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonamide
CAS RN [98967-40-9] Development codes DE-498; XRD-498 (both Dow)
PHYSICAL CHEMISTRY
Mol. wt. 325.3 M.f. C12H9F2N5O2S Form Off-white, odourless solid. M.p. 251-253 ºC V.p. 3.7 ´ 10-7 mPa (25 ºC) KOW logP = -0.68 (25 ºC, unstated pH) S.g./density 1.77 (21 ºC) Solubility In water 49 mg/l (pH 2.5); solubility increases with pH. Very slightly soluble in acetone and methanol. Insoluble in hexane and xylene. Stability Aqueous photolysis DT50 6-12 mo. Soil photolysis DT50 3 mo. pKa 4.6 F.p. >93 °C
COMMERCIALISATION
Manufacturers Dow AgroSciences
APPLICATIONS
Biochemistry Branched chain amino acid (leucine, isoleucine and valine) synthesis (ALS or AHAS) inhibitor. Selectivity in soya beans is due to rapid metabolic deactivation. Mode of action Systemic herbicide, absorbed by roots and leaves of plants and translocated to growth points. Uses Used alone, at 25-78 g/ha, and in combination with trifluralin or metolachlor for control of broad-leaved weeds and grasses in soya beans, field peas and maize. Phytotoxicity Crops damaged by soil application of flumetsulam include sugar beet, cotton, oilseed rape, grain sorghum, tomatoes, and sunflowers. Formulation types OF; SC; WG. Selected products: 'Broadstrike' (Dow AgroSciences)
OTHER PRODUCTS
'Preside' (Dow AgroSciences); 'Python' (Dow AgroSciences); 'Scorpion' (Dow AgroSciences) mixtures: 'Broadstrike Plus' (+ clopyralid) (Dow AgroSciences); 'Frontrow' (+ cloransulam-methyl) (Dow AgroSciences); 'Hornet' (+ clopyralid) (clopyralid as potassium salt) (Dow AgroSciences); 'Striker' (+ clopyralid) (clopyralid as potassium salt) (Dow AgroSciences); 'Accent Gold' (+ nicosulfuron+ rimsulfuron+ clopyralid) (DuPont); 'Bicep Magnum TR' (+ S-metolachlor+ atrazine) (Syngenta) Discontinued products mixtures: 'Broadstrike Post' * (+ 2,4-D+ clopyralid) (Dow AgroSciences); 'Scorpion III' * (+ 2,4-D+ clopyralid) (Dow AgroSciences); 'Broadstrike Dual' * (+ metolachlor) (Syngenta)
ANALYSIS
By glc with mass selection detection.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000 mg/kg. Slightly irritating to eyes (rabbits). Non-sensitising to skin (guinea pigs). Inhalation LC50 (4 h) for rats 1.2 mg/l. NOEL for mice >1000, female rats 500, male rats 1000, dogs 1000 mg/kg b.w. Other Non-teratogenic (dietary) in rats. Non-mutagenic in the Ames test. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2250 mg/l. Dietary LC50 (8 d) for bobwhite quail and mallard ducks >5620 mg/l. Fish LC50 (96 h) for silverside minnow >379 mg/l. Non-toxic to fathead minnow and bluegill sunfish. Daphnia Non-toxic. Algae EC50 (5 d) for green algae (Selenastrum capricornutum) 4.9 mg/l, bluegreen algae (Anabaena flos-aquae) 167 mg/l. Other aquatic spp. LC50 for shrimp >349 mg/l. Bees LC50 >100 mg/bee. NOEL 36 mg/bee. Worms LC50 (14 d) >950 mg/kg soil.
ENVIRONMENTAL FATE
Animals Rapidly cleared via urine and faeces with no metabolites in most mammals. 5-Hydroxy metabolite found in kidney tissue in the hen. Plants DT50 in maize 2 h, soya beans 18 h, Chenopodium 131 h. Metabolites depend on the species; 5-hydroxy or 5-methoxy derivatives are common. Soil/Environment Availability of flumetsulam in soil is principally dependent upon soil pH and organic matter. Herbicidal activity increases as pH increases and organic matter decreases. DT50 in soil (25 ºC, pH ³7, o.m. content <4%; or pH 6-7, o.m. content c. 1%) £1 mo. DT50 in soil (pH 6-7, o.m. content 2-4%) 1-2 mo. Koc 5 - 182; Kd 0.05 - 2.4.
|