|
flucarbazone-sodium
Herbicide
HRAC B WSSA 2; sulfonylaminocarbonyltriazolinone
NOMENCLATURE
Common name flucarbazone-sodium (BSI, pa ISO)
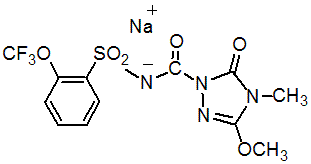
IUPAC name 4,5-dihydro-3-methoxy-4-methyl-5-oxo-N-(2-trifluoromethoxyphenylsulfonyl)-1H-1,2,4-triazole-1-carboxamide sodium salt
Chemical Abstracts name 4,5-dihydro-3-methoxy-4-methyl-5-oxo-N-[[2-(trifluoromethoxy)phenyl]sulfonyl]-1H-1,2,4-triazole-1-carboxamide, sodium salt
CAS RN [181274-17-9]; (acid [145026-88-6]) Development codes MKH 6562 (Bayer); SJO 0498
PHYSICAL CHEMISTRY
Mol. wt. 418.3 M.f. C12H10F3N4NaO6S Form Colourless, odourless, crystalline powder. M.p. 200 °C (decomp.) V.p. <1 ´ 10-6 mPa (20 °C, calc.) KOW logP = -0.89 (pH 4), -1.85 (pH 7), -1.89 (pH 9), -2.80 (unbuffered) (all 20 °C) Henry <1 ´ 10-11 Pa m3 mol-1 (20 °C, calc.) S.g./density 1.59 g/ml Solubility In water 44 g/l (pH 4-9, 20 °C). pKa 1.9
COMMERCIALISATION
History Reported by H. J. Santel et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1999, 1, 23). Introduced in 2000 by Bayer AG. Rights acquired by Arvesta in 2002. Manufacturers Bayer CropScience
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Mode of action Absorbed through foliage and roots, and translocated acropetally and basipetally. Uses For post-emergence control of grass weeds, especially Avena fatua and Setaria viridis, and some broad-leaved weeds, in wheat; applied at 21 g/ha. Formulation types WG. Selected products: 'Everest' (Arvesta)
OTHER PRODUCTS
'Vulcano' (Bayer CropScience, Arvesta)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >5000 mg/kg. Non-irritating to skin; slightly/moderately irritating to eyes (rabbits). Not a skin sensitiser (guinea pigs). Inhalation LC50 (acute) for rats >5.13 mg/l. NOEL (2 y) for rats 125, mice 1000 mg/kg diet; (1 y) for female dogs 200, male dogs 1000 mg/kg diet. ADI 0.04 mg/kg b.w. (Bayer proposed). Other No evidence for any neurotoxic, genotoxic, teratogenic or carcinogenic potential. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Sub-acute dietary LC50 for bobwhite quail >5000 ppm. Fish LC50 (96 h) for bluegill sunfish >99.3, rainbow trout >96.7 mg/l. Daphnia EC50 (48 h) >109 mg/l. Algae EC50 for Selenastrum capricornutum 6.4 mg/l. Other aquatic spp. EC50 for Lemna gibba 0.0126 mg/l. Bees Non-toxic to honeybees. Worms LC50 for earthworms >1000 mg/kg.
ENVIRONMENTAL FATE
Animals Almost completely excreted via faeces and urine within 48 h of oral administration to rats. Plants Extensively metabolised in wheat plants. The relevant residues for enforcement purposes are the parent compound and the N-desmethyl metabolite. Soil/Environment Average soil DT50 17 d. Photolytic DT50 in soil and water >500 d. Not mobile in soil; in dissipation studies, no product residues detected below 30 cm.
|