|
ethalfluralin
Herbicide
HRAC K1 WSSA 3; dinitroaniline
NOMENCLATURE
Common name ethalfluralin (BSI, E-ISO, ANSI, WSSA); éthalfluraline ((f) F-ISO)
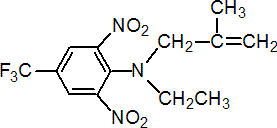
IUPAC name N-ethyl-a,a,a-trifluoro-N-(2-methylallyl)-2,6-dinitro-p-toluidine
Chemical Abstracts name N-ethyl-N-(2-methyl-2-propenyl)-2,6-dinitro-4-(trifluoromethyl)benzenamine
CAS RN [55283-68-6] EEC no. 259-564-3 Development codes EL-161 (Lilly)
PHYSICAL CHEMISTRY
Mol. wt. 333.3 M.f. C13H14F3N3O4 Form Yellow-orange crystals, with a faint amine odour. M.p. 55-56 ºC B.p. Decomposes at 256 ºC V.p. 11.7 mPa (25 ºC) KOW logP = 5.11 (pH 7, 25 ºC) Henry 1.30 ´ 101 Pa m3 mol-1 (25 °C, calc.) Solubility In water 0.3 mg/l (pH 7, 25 ºC). In acetone, acetonitrile, benzene, chloroform, dichloromethane, xylene >500, methanol 82-100 (all in g/l, 25 ºC). Stability Tech. stable at 52 ºC (highest storage temperature tested). No hydrolysis after 33 d at pH 3, 6 and 9 (51 ºC). Photolysis DT50 (aqueous) 6.3 h; (vapour phase) 2 h. F.p. 151 °C (Setaflash closed cup; ASTM D-3278)
COMMERCIALISATION
History Herbicide reported by G. Skylakakis et al. (Proc. Br. Weed Control Conf., 12th, 1974, 2, 795). Introduced in Turkey (1974) by Eli Lilly & Co. (agrochemical interests now Dow AgroSciences). Patents US 3257190 Manufacturers Dintec
APPLICATIONS
Biochemistry Microtubule assembly inhibition. Mode of action Selective soil-herbicide, which affects seed germination and associated physiological growth processes. No significant absorption or translocation occurs in crops grown in soil treated with ethalfluralin. Uses Control of most germinating annual grasses and broad-leaved weeds in cotton, soya beans, dry beans, lentils, peanuts, cucurbits, safflowers, and sunflowers. Applied pre-plant soil-incorporated at 1.0-1.25 kg/ha or, for peanuts and cucurbits, by post-planting surface application. Used in combination with atrazine for herbicidal control in maize and sorghum. Formulation types EC; GR; WG. Selected products: 'Edge' (Dow AgroSciences); 'Sonalan' (Dow AgroSciences); 'Sonalen' (Dow AgroSciences)
OTHER PRODUCTS
'Curbit' (Platte)
ANALYSIS
Product analysis by glc with FID or by spectrophotometry (E. W. Day, Anal. Methods Pestic. Plant Growth Regul., 1978, 10, 341). Residues determined by glc with ECD (idem, ibid.). Details available from Dow AgroSciences.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >5000 mg/kg. Moderate to severe irritant to skin; moderate eye irritant (rabbits). Inhalation LC50 (1 h) for rats >2.8 mg/l. NOEL In 2 y feeding trials, rats and mice receiving 100 mg/kg diet (for rats 4.2, mice 10.3 mg/kg b.w. daily) showed no ill-effects. ADI 0.042 mg/kg. Other Non-mutagenic. Toxicity class WHO (a.i.) U; EPA (formulation) II
ECOTOXICOLOGY
Birds Acute oral LD50 for bobwhite quail >2000 mg/kg. Dietary LC50 (5 d) for bobwhite quail and mallard ducks >5000 mg/kg diet. Fish LC50 (96 h) for bluegill sunfish 0.102, rainbow trout 0.136 mg/l. Daphnia LC50 (48 h) >0.365 mg/l; NOEC (21 d) 0.068 mg/l. Algae For Selenastrum capricornutum, NOEL 0.004 mg/l; EC50 specific growth rate 0.009 mg/l. Other aquatic spp. EC50 (shell deposition) for eastern oyster (Crassostrea virginica) 0.172 mg/l. Bees Not toxic to bees. LD50 (contact) 51 mg/bee (by linear regression).
ENVIRONMENTAL FATE
Animals In rats, following oral administration, 86% is excreted within 48 hours (64% in the faeces and 22% in the urine) and 95% within 7 days. In the urine, 3 metabolites have been identified, resulting from oxidation of the N-alkyl side-chain and/or by dealkylation. Glucuronide conjugates have been found as metabolites in the bile. Plants The same pattern of metabolism is observed as for trifluralin. Soil/Environment Ethalfluralin is strongly adsorbed on soil, with negligible leaching; half-life in soils 25-46 days. Photolytic and microbial degradation takes place (B. J. Hayden & A. E. Smith, Bull. Environ. Contam. Toxicol., 1983, 25, 508). Aerobic metabolism in sandy loam soil (EPA laboratory studies) DT50 45 d; more rapid metabolism anaerobically (DT50 14 d) in same soil; soil photolysis DT50 14 d. Aquatic DT50 (anaerobic) 38.3 h. Koc 4100-8400; Kd 11.9 to 97 with o.m. 0.5 to 2.0%.
|