|
diflubenzuron
Insecticide
IRAC 15; benzoylurea
NOMENCLATURE
Common name diflubenzuron (BSI, E-ISO, (m) F-ISO, ANSI, ESA)
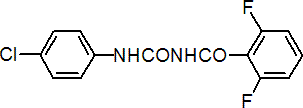
IUPAC name 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)urea
Chemical Abstracts name N-[[(4-chlorophenyl)amino]carbonyl]-2,6-difluorobenzamide
Other names DFB CAS RN [35367-38-5] Development codes DU 112307; PH 60-40; PDD 60-40-I (all Duphar); TH 6040 (T. H. Agriculture & Nutrition Co., Inc.) Official codes OMS 1804; ENT 29 054
PHYSICAL CHEMISTRY
Composition Tech. grade diflubenzuron is ³95% pure. Mol. wt. 310.7 M.f. C14H9ClF2N2O2 Form Colourless crystals; (tech., off-white to yellow crystals). M.p. 228 °C; (tech., 210-230 ºC, decomp.) V.p. 1.2 ´ 10-4 mPa (25 ºC) (gas saturation method) KOW logP = 3.89 S.g./density 1.56 Solubility In water 0.08 mg/l (pH 7, 25 ºC). In n-hexane 0.063, toluene 0.29, dichloromethane 1.8, methanol 1.1 (all in g/l, 20 °C). Stability Light-sensitive when in solution, but stable to sunlight as a solid. <0.5% decomposition after 1 d storage at 100 ºC; <0.5% after 7 d at 50 ºC. In aqueous solution (20 ºC), stable at pH 5 and 7 (DT50 >150 d), at pH 9 DT50 42 d.
COMMERCIALISATION
History Insecticide reported by J. J. van Daalen et al. (Naturwissenschaften, 1972, 59, 312) and reviewed by A. C. Grosscurt (Pestic. Sci., 1978, 9, 373) and W. Maas et al. (Chem. Pflanzenschutz-Schädlingsbekämpfungsmittel, 1980, 6, 423). Introduced in 1975 by Philips-Duphar B.V. (now Crompton Corp.). Patents GB 1324293; US 3748356; US 3989842 Manufacturers Crompton; Sharda; Sundat
APPLICATIONS
Biochemistry Chitin synthesis inhibitor; and so interferes with the formation of the insect cuticle. This action is quite specific; related biochemical processes, such as chitin synthesis in fungi, and biosynthesis of hyaluronic acid and other mucopolysaccharides in chickens, mice and rats are not affected. Mode of action Non-systemic insect growth regulator with contact and stomach action. Acts at time of insect moulting, or at hatching of eggs. Uses For control of a wide range of leaf-eating insects in forestry, woody ornamentals and fruit. Controls certain major pests in cotton, soya beans, citrus, tea, vegetables and mushrooms. Also controls larvae of flies, mosquitoes, grasshoppers and migratory locusts. Used as an ectoparasiticide on sheep for control of lice, fleas and blowfly larvae. Due to its selectivity and rapid degradation in soil and water, diflubenzuron has no or only a slight effect on the natural enemies of various harmful insect species. These properties make it suitable for inclusion in integrated control programmes. Diflubenzuron is effective at 25-75 g/ha against most leaf-feeding insects in forestry; in concentrations of 0.01-0.015% a.i. against codling moth, leaf miners and other leaf-eating insects in top fruit; in concentrations of 0.0075-0.0125% a.i. against citrus rust mite in citrus; and at a dosage of 50-150 g/ha against a number of pests in cotton (cotton boll weevil, armyworms, leafworms), soya beans (soya bean looper complex) and maize (armyworms). Also for control of larvae of mushroom flies in mushroom casing (1 g/m2); mosquito larvae (25-100 g/ha); fly larvae in animal housings (0.5-1 g/m2 surface); and locusts and grasshoppers (60-67.5 g/ha). Formulation types GR; HN; OF; SC; UL; WG; WP. Compatibility Incompatible with strongly alkaline products. Selected products: 'Dimilin' (Crompton); 'Hilmilin' (Hindustan); 'Kitinex' (Cequisa); 'Patron' (Vapco)
OTHER PRODUCTS
'Adept' (Crompton); 'Micromite' (Crompton); 'Astonex' (Shell)
ANALYSIS
Product analysis by rplc (CIPAC Handbook, 1998, H, 141; AOAC Methods, 17th Ed., 983.07; A. van Rossum et al., Anal. Methods Pestic. Plant Growth Regul., 1984, 13, 165). Residues determined by hplc or glc after hydrolysis to 4-chloroaniline which is converted to a derivative (B. Rabenort et al., ibid., 1978, 10, 57).
MAMMALIAN TOXICOLOGY
Reviews CAG; FAO/WHO 92, 94 (see part 2 of Bibliography). Oral Acute oral LD50 for rats and mice >4640 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >2000, rats >10 000 mg/kg. Not a skin irritant; slight eye irritant (rabbits). Not a skin sensitiser (guinea-pigs). Inhalation LC50 for rats >2.88 mg/l. NOEL (2 y) for rats 40 mg/kg diet. No teratogenic, mutagenic, or oncogenic effect was observed. ADI (JMPR) 0.02 mg/kg b.w. [2001]; 0.02 (JECFA evaluation) [1994]. Other Acute i.p. LD50 for mice >2150 mg/kg. Toxicity class WHO (a.i.) U; EPA (formulation) III
ECOTOXICOLOGY
Birds Dietary LC50 (8 d) for bobwhite quail and mallard ducks >4640 mg/kg diet. Fish LC50 (96 h) for zebra fish (Brachydanio rerio) >0.2,rainbow trout >0.2 mg/l. Daphnia LC50 (48 h) 7.1 mg/l. Algae LC50 for Selenastrum capricornutum >0.3 mg/l. Other aquatic spp. LC50 for molluscs >200 mg/l. Bees Not hazardous to bees and predatory insects; LD50 (oral and contact) >100 mg/bee. Worms NOEC for Eisenia foetida 780 mg/kg substrate.
ENVIRONMENTAL FATE
EHC 184 (WHO, 1996). Animals In rats, following oral administration, elimination is partly as the unchanged parent compound in the faeces, partly as hydroxylated metabolites (for c. 80%) and as 4-chlorophenylurea plus 2,6-difluorobenzoic acid (for c. 20%). The intestinal absorption is strongly related to the dosage administered - the higher the dosage, the more (relatively) is excreted unchanged in the faeces. See A. Verloop & C. D. Ferrell, ACS Symp. Series, 1977, No. 37, 237. Plants Non-systemic. Not metabolised on plants (idem, ibid.). Soil/Environment Diflubenzuron is strongly absorbed by soil/humic acid complex and is virtually immobile in soil. Rapidly degraded in soil, with a half-life of <7 days. The principal degradation products are 4-chlorophenylurea and 2,6-difluorobenzoic acid (idem, ibid.).
|