|
cyclosulfamuron
Herbicide
HRAC B WSSA 2; sulfonylurea
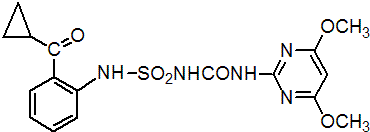
NOMENCLATURE
Common name cyclosulfamuron (pa ISO, ANSI)
IUPAC name 1-[2-(cyclopropylcarbonyl)phenylsulfamoyl]-3-(4,6-dimethoxypyrimidin-2-yl)urea
Chemical Abstracts name N-[[[2-(cyclopropylcarbonyl)phenyl]amino]sulfonyl]-N'-(4,6-dimethoxy-2-pyrimidinyl)urea
CAS RN [136849-15-5] Development codes AC 322,140 (Cyanamid)
PHYSICAL CHEMISTRY
Mol. wt. 421.4 M.f. C17H19N5O6S Form Off-white solid. M.p. 160.9-162.9 °C; (tech., 149.6-153.2 °C) V.p. c. 2.2 ´ 10-2 mPa (20 °C, gas saturation method) KOW logP = 1.58 (pH 3), 2.045 (pH 5), 1.69 (pH 6), 1.41 (pH 7), 0.7 (pH 8) (all 25 ºC) S.g./density 0.624 (20 °C) Solubility In water 0.17 (pH 5), 6.52 (pH 7), 549 (pH 9) (all in ppm, 25 ºC). Stability DT50 0.33 (pH 5), 1.68 (pH 7), 1.66 (pH 9) (all in days). Stable 18 months (25 °C), 12 months (36 °C), 3 months (45 °C). pKa 5.04
COMMERCIALISATION
History Reported by M. E. Condon et al. (Proc. Br. Crop Prot. Conf. - Weeds, 1993, 1, 41). Introduced in August 1997 by BASF; combination products with Bayer, Kaken, and Chugai. Patents US 5009699 Manufacturers BASF
APPLICATIONS
Biochemistry Branched chain amino acid synthesis (ALS or AHAS) inhibitor. Acts by inhibiting biosynthesis of the essential amino acids valine and isoleucine, hence stopping cell division and plant growth. Contributing factors for herbicide safety in rice, wheat and barley are herbicide and crop placement, metabolic inactivation in the shoot, and the ability to translocate the herbicide to sites of potential metabolic inactivation (S. J. Rodaway et al., Proc. Br. Crop Prot. Conf. - Weeds, 1993, 1, 239). Uses Control of a variety of dicotyledonous and sedge weeds, e.g. Cyperus serotinus, Eleocharis kuroguwai and Sagittaria pygmaea in rice, and Galium aparine, Matricaria spp., Veronica spp., Sinapis arvensis and Brassica napus in wheat and barley at 25-50 g/ha, and in rice at 45-60 g/ha. Formulation types GR; WP. Selected products: 'Invest' (BASF); 'Jin-Qiu' (BASF); 'Orysa' (BASF)
OTHER PRODUCTS
'Ichiyonmaru' (BASF) mixtures: 'Nebiros' (+ daimuron+ cafenstrole) (BASF); 'Utopia' (+ pentoxazone) (BASF, Kaken); 'Agalia' (+ daimuron+ fentrazamide) (Bayer CropScience, BASF); 'Bigsure' (+ daimuron+ fentrazamide) (Bayer CropScience, BASF); 'Naegamae' (+ fentrazamide) (Misung)
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats and mice >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rabbits >4000 mg/kg. Non-irritating to skin, and mildly irritating to eyes (rabbits). Inhalation LC50 (4 h) for rats >5.2 mg/l. NOEL (2 y) for rats 1000 mg/kg diet (50 mg/kg b.w. daily); (1 y) for dogs 100 mg/kg diet (3 mg/kg b.w. daily). Other Non-mutagenic in the Ames test. Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Acute oral LC50 for quail >1880 mg/kg. Dietary LC50 (8 d) for quail >5010 mg/kg. Fish LC50 (72 h) for carp >50 ppm; (96 h) for trout >7.7, bluegill >8.2 mg/l. Daphnia LC50 (48 h) >9.1 mg/l. Algae EC50 (72 h) 0.44 mg/l. Bees Acute LD50 (24 h) (oral) >99 mg/bee; (contact) >106 mg/bee. Worms No adverse effects at 892 mg/kg.
ENVIRONMENTAL FATE
Animals In rats, cyclosulfamuron is rapidly absorbed and readily excreted, mainly via the faeces. Plants In crop plants, by hydrolysis of the urea bridge, giving inactive compounds. See Biochemistry. Soil/Environment Koc (ave) in 4 U.S. soils and 4 Japanese paddy soils is 1440.
|