|
clofencet
Plant growth regulator
NOMENCLATURE
clofencet
Common name clofencet (pa ISO, ANSI)
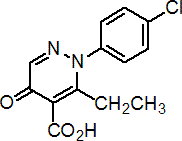
IUPAC name 2-(4-chlorophenyl)-3-ethyl-2,5-dihydro-5-oxopyridazine-4-carboxylic acid
Chemical Abstracts name 2-(4-chlorophenyl)-3-ethyl-2,5-dihydro-5-oxo-4-pyridazinecarboxylic acid
CAS RN [129025-54-3] Development codes MON 21200 (Monsanto)
clofencet-potassium
IUPAC name potassium 2-(4-chlorophenyl)-3-ethyl-2,5-dihydro-5-oxopyridazine-4-carboxylate
Chemical Abstracts name potassium 2-(4-chlorophenyl)-3-ethyl-2,5-dihydro-5-oxo-4-pyridazinecarboxylate
CAS RN [82697-71-0] Development codes MON 21233 (Monsanto); RH 754 (Rohm & Haas); RH0754 (Rohm & Haas); FC 4001 (Zeneca); ICIS 0754 (ICI)
PHYSICAL CHEMISTRY
clofencet
Mol. wt. 278.7 M.f. C13H11ClN2O3 pKa 2.83 (20 °C)
clofencet-potassium
Mol. wt. 316.8 M.f. C13H10ClKN2O3 M.p. 269 °C (decomp.) V.p. <1 ´ 10-2 mPa (25 °C) KOW logP = -2.2 (25 °C) Henry <5.7 ´ 10-9 Pa m3 mol-1 (25 °C, calc.) S.g./density 1.44 (20 °C) Solubility In water >552; >655 (pH 5), >696 (pH 7), >658 (pH 9) (all in g/l, 23 °C). In methanol 16, acetone <0.5, dichloromethane <0.4, toluene <0.4, ethyl acetate <0.5, n-hexane <0.6 (all in g/l, 24 °C). Stability Stable 14 d (54 °C). Stable to hydrolysis in sterile aqueous pH 5, 7, and 9 buffered solutions. Moderately persistent to aqueous photolysis; DT50 increases with pH.
COMMERCIALISATION
History Discovered by Rohm and Haas Co. (now Dow AgroSciences). Introduced by Monsanto Co. for use in wheat in 1997.
APPLICATIONS
clofencet-potassium
Mode of action Systemic. Inhibits pollen formation. Uses The potassium salt is a chemical hybridising agent for wheat. Applied at 3-5 kg/ha. Formulation types SC. Selected products: 'Genesis' (Monsanto); 'Detasselor' (Syngenta, Monsanto)
MAMMALIAN TOXICOLOGY
clofencet
ADI 0.06 mg/kg.
clofencet-potassium
Oral Acute oral LD50 for male rats 3437, female rats 3150 mg/kg. Skin and eye Acute dermal LD50 for rats >5000 mg/kg. Eye irritant; not a skin irritant (rabbits). Not a skin sensitiser (guinea pigs). Inhalation EC50 for rats >3.8 mg/l. NOEL (1 y) for dogs 5.0 mg/kg daily.
ECOTOXICOLOGY
clofencet-potassium
Birds Acute oral LD50 for ducks >2000, quail 1414 mg/kg. Dietary LC50 for ducks and quail >4818 ppm. Fish LC50 for bluegill sunfish >1070, rainbow trout >990 ppb. Daphnia EC50 >1193 mg/l. Algae EbC50 (96 h) for Selenastrum capricornutum 141 mg/l; ErC50 374 mg/l. Other aquatic spp. IC50 (14 d) for Lemna gibba (G3) >6.1 mg/l. Bees LD50 (contact and oral) >100 mg/bee. Worms EC50 >1000 ppm.
ENVIRONMENTAL FATE
Animals In rats, clofencet was rapidly absorbed and excreted; >78% of the administered 14C-label was eliminated in the urine within 24 h. Clofencet was eliminated mostly unmetabolised and mainly in the urine. At 7 days post-dosing, <1% was retained in the tissue. Plants Clofencet is poorly metabolised in wheat, >80% of the residue in wheat seed, and c. 70% of the residue in wheat straw is unmetabolised clofencet. Soil/Environment Metabolism of clofencet in laboratory soil studies is very slow. After one year in loamy sand (pH 6.0, 4.5% o.m.) and silt loam (pH 7.7, 2.4% o.m.), c. 70% of the applied clofencet remains; the main route of dissipation is soil binding. Clofencet is fairly stable to soil photolysis (74-81% of parent clofencet remains after 30-32 d). DT50 20-28 days in aqueous solutions (pH 5, 7 and 9). In European field dissipation studies, DT50 in silty clay and silty clay loam soils (pH 6.6-7.8) 25-66 days for three sites, although slower degradation was seen at some sites. Batch equilibrium studies and pKa considerations suggest that clofencet is very mobile in sand, loamy sand, and silt loam soils, but laboratory and field studies suggest that the significant soil binding which occurs with time mitigates the tendency towards mobility. Clofencet has the potential for runoff or soil mobility but, in practice, because of specialised use and stewardship practices, effects of mobility and persistence are less likely to be significant.
|