|
chlorotoluron
Herbicide
HRAC C2 WSSA 7; urea
NOMENCLATURE
Common name chlorotoluron (BSI (from 1984), E-ISO, (m) F-ISO); chlortoluron (BSI (before 1984), (m) France, New Zealand)
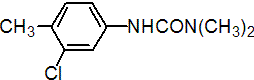
IUPAC name 3-(3-chloro-p-tolyl)-1,1-dimethylurea
Chemical Abstracts name N'-(3-chloro-4-methylphenyl)-N,N-dimethylurea
CAS RN [15545-48-9] EEC no. 239-592-2 Development codes C 2242 (Ciba)
PHYSICAL CHEMISTRY
Mol. wt. 212.7 M.f. C10H13ClN2O Form White powder. M.p. 148.1 ºC V.p. 0.005 mPa (25 °C) KOW logP = 2.5 (25 ºC) Henry 1.44 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.40 g/cm3 (20 ºC) Solubility In water 74 mg/l (25 ºC). In acetone 54, dichloromethane 51, ethanol 48, toluene 3.0, hexane 0.06, n-octanol 24, ethyl acetate 21 (all in g/l, 25 ºC). Stability Stable to heat and u.v. light. Slowly hydrolysed by strong acids and alkalis. DT50 (calc.) >200 d (pH 5, 7, 9; 30 ºC).
COMMERCIALISATION
History Herbicide reported by Y. L'Hermite et al. (C. R. Journ. Etud. Herbic. Conf., COLUMA, 5th, 1969, II, 349). Introduced by Ciba AG (now Syngenta AG). Patents BE 728267; GB 1255258 Manufacturers ÉMV; Jingma; Makhteshim-Agan; Nufarm GmbH; Syngenta; Synthesia; United Phosphorus
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Selective herbicide, absorbed by the roots and foliage. Uses Effective at 1.5-3.0 kg/ha, both as a residual soil-acting herbicide and a contact foliar-spray, against many broad-leaved and grass weeds of winter cereals, especially against Alopecurus myosuroides. It is combined with mecoprop to improve the control of Galium, Papaver and Veronica spp. Phytotoxicity Some varieties of wheat and barley may be injured. Formulation types FW; GR; SC; WP. Selected products: 'Dicuran' (Syngenta); 'Chlortophyt' (Agriphar); 'Lentipur' (Nufarm GmbH, Nufarm UK); 'Tolurex' (Makhteshim-Agan)
OTHER PRODUCTS
'Alert' (Crystal); 'Atol' (Nufarm UK); 'Cekutoluron' (Cequisa); 'Chlortosint' (Nufarm GmbH); 'Clorturex' (Aragro); 'Cloturex' (Aragro); 'Copal' (DAPT); 'Monsun' (Stefes); 'NWA CTU 500' (Nufarm UK); 'Syncuran' (Synthesia); 'Tolugan 700' (Makhteshim-Agan); 'Tolurane' (Diachem) mixtures: 'Dicuran Forte' (+ triasulfuron) (Syngenta); 'Tricuran' (+ terbutryn+ triasulfuron) (Syngenta); 'Tolugan Extra' (+ isoproturon) (Makhteshim-Agan); 'Tomcat' (+ terbutryn) (Probelte) Discontinued products: 'Dicurane' * (Ciba); 'Ashlade Tol-7' * (Ashlade); 'Ludorum' * (Tripart); 'Toluron' * (Stefes); 'Toro' * (Sipcam) mixtures: 'Totem' * (+ pendimethalin) (Cyanamid)
ANALYSIS
Product analysis by determination of dimethylamine produced on hydrolysis, or by tlc (AOAC Methods, 17th Ed., 977.06; CIPAC Handbook, 1980, 1A, 1151). Residues determined by hplc or by alkaline hydrolysis to 3-chloro-p-toluidine, a derivative of which is determined by glc with TID, MCD, or ECD. Details available from Syngenta.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for rats >5000 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2000 mg/kg; not irritant to skin and eyes of rabbits. Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h) for rats >5.3 mg/l. NOEL (2 y) for rats 100 ppm (5 mg/kg daily), mice 100 ppm (11.3 mg/kg daily). Water GV 30 mg/l (TDI 11.3 mg/kg b.w.). Toxicity class WHO (a.i.) U
ECOTOXICOLOGY
Birds Dietary LC50 (8 d) for mallard ducks >6800, Japanese quail >2150, pheasants >10 000 ppm. Fish LC50 (96 h) for rainbow trout 35, bluegill sunfish 50, crucian carp >100, catfish 60, guppy >49 mg/l. Daphnia LC50 (48 h) 67 mg/l. Algae EC50 (72 h) for Scenedesmus subspicatus 0.024 mg/l. Bees LD50 (48 h) (contact) >100 mg/bee; (ingestion) >1000 ppm. Worms LC50 for earthworms >1000 mg/kg.
ENVIRONMENTAL FATE
Animals In mammals, following oral administration, >90% is eliminated in the urine and faeces within 24 hours. Main metabolism is via N-demethylation and stepwise oxidation of the ring methyl group to hydroxymethyl and carboxymethyl derivatives. Plants Metabolites found in winter wheat include 3-chloro-p-toluidine, 3-(3-chloro-4-methylphenyl)-1-methylurea, and 1-(3-chloro-4-methylphenyl)urea. Soil/Environment DT50 in soil 30-40 d, in water >200 d.
|