|
carfentrazone-ethyl
Herbicide
HRAC E WSSA 14; triazolinone
NOMENCLATURE
Common name carfentrazone-ethyl (BSI, pa ISO, ANSI)
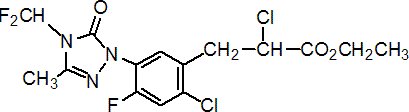
IUPAC name ethyl (RS)-2-chloro-3-[2-chloro-5-(4-difluoromethyl-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl)-4-fluorophenyl]propionate
Chemical Abstracts name ethyl a,2-dichloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl]-4-fluorobenzenepropanoate
CAS RN [128621-72-7] (for the acid); [128639-02-1] (for the ethyl ester) Development codes F8426; F116426 (both FMC)
PHYSICAL CHEMISTRY
Mol. wt. 412.2 M.f. C15H14Cl2F3N3O3; C13H10Cl2F3N3O3 for acid Form Viscous yellow liquid. M.p. -22.1 ºC B.p. 350-355 ºC/760 mmHg V.p. 1.6 ´ 10-2 mPa (25 ºC) KOW logP = 3.36 Henry 2.47 ´ 10-4 Pa m3 mol-1 (20 °C, calc.) S.g./density 1.457 (20 ºC) Solubility In water 12 mg/ml (20 ºC), 22 mg/ml (25 ºC), 23 mg/ml (30 ºC). In toluene 0.9, hexane 0.03 (both in g/ml, 20 °C); miscible with acetone, ethanol, ethyl acetate and dichloromethane. Stability Hydrolytic DT50 3.6 h (pH 9), 8.6 d (pH 7), stable (pH 5). Aqueous photolytic DT50 8 d. F.p. >110 ºC
COMMERCIALISATION
History Reported by W. A. van Saun et al. (Proc. Br. Crop Prot. Conf. - Weeds,1993, 1, 19). Introduced in 1997. Manufacturers FMC
APPLICATIONS
Biochemistry Acts by inhibition of protoporphyrinogen oxidase, leading to membrane disruption. Mode of action Absorbed by foliage, with limited translocation. Uses Post-emergence control in cereals of a wide range of broad-leaved weeds, especially Galium aparine, Abutilon theophrasti, Ipomoea hederacea var. hederacea, Chenopodium album and several mustard species, at 9-35 g/ha. Also for desiccation of potatoes, at 60 g/ha. Phytotoxicity Good tolerance in wheat, barley and rice. Formulation types EC; SG; WG. Selected products: 'Aurora' (FMC); 'Spotlight' (FMC); mixtures: 'Affinity' (+ isoproturon) (FMC); 'Platform S' (+ mecoprop-P) (FMC)
OTHER PRODUCTS
'Aim' (FMC); 'Platform' (FMC); 'Shark' (FMC) mixtures: 'Alli?Express' (+ metsulfuron-methyl) (DuPont); 'Ally Express' (+ metsulfuron-methyl) (DuPont); 'Aurora Turbo' (+ mecoprop-P) (Philagro); 'Harmony Express' (+ thifensulfuron-methyl) (DuPont); 'Lexus Class' (+ flupyrsulfuron-methyl-sodium) (DuPont) Discontinued products mixtures: 'Affinity' * (+ isoproturon) (DuPont); 'Platform S' * (+ mecoprop-P) (DuPont)
ANALYSIS
Details from FMC.
MAMMALIAN TOXICOLOGY
Oral Acute oral LD50 for female rats 5143 mg/kg. Skin and eye Acute percutaneous LD50 for rats >4000 mg/kg. Minimally irritating to eyes and non-irritating to skin (rabbits). No skin sensitisation (guinea pigs). Inhalation LC50 (4 h) for rats >5 mg/l. NOEL (2 y) for rats 3 mg/kg daily. ADI 0.03 mg/kg (proposed). Other Non-mutagenic in the Ames test. Toxicity class WHO (a.i.) III (company classification); EPA (formulation) III, IV EC classification N; R50, R53
ECOTOXICOLOGY
Birds LD50 for quail >1000 mg/kg. LC50 for quail and ducks >5000 ppm. Fish LC50 (96 h) 1.6-43 mg/l, depending on species. Daphnia EC50 (48 h) 9.8 mg/l. Algae EC50 12-18 mg/l, depending on species. Other aquatic spp. EC50 (96 h) for eastern oyster 2.05, mysid shrimp 1.16 ppm. Bees LD50 (oral) >35; (contact) >200 mg/bee. Worms LC50 >820 mg/kg soil.
ENVIRONMENTAL FATE
Animals In rats, c. 80% of the administered dose is rapidly absorbed and excreted in the urine within 24 h. The major metabolite was the corresponding acid. Further metabolism appears to involve oxidative hydroxylation of the methyl group or dehydrochlorination to form the corresponding cinnamic acid. Plants Rapidly converted to the free acid, which is hydroxylated and then oxidised at the triazolinone methyl to form the dibasic acid; DT50 (carfentrazone-ethyl) <7 d, DT50 (carfentrazone) <28 d. Soil/Environment Broken down in the soil by microbial action; not susceptible to photodecomposition nor volatility following soil application. Strongly adsorbed to sterile soils (Koc 750?0 at 25 ºC). In non-sterile soils, rapidly converted to the free acid, which has low soil binding (Koc 15-35 at 25 ºC, pH 5.5). In the laboratory, soil DT50 is a few hours, degrading to the free acid, which in turn has DT50 2.5-4.0 d.
|