|
bentazone
Herbicide
HRAC C3 WSSA 6; benzothiadiazinone
NOMENCLATURE
bentazone
Common name bentazone (BSI, E-ISO, (f) F-ISO, JMAF); bentazon (ANSI, Canada, WSSA); bendioxide (Republic of South Africa)
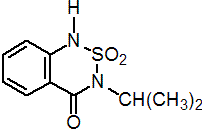
IUPAC name 3-isopropyl-1H-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide
Chemical Abstracts name 3-(1-methylethyl)-1H-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide
CAS RN [25057-89-0] EEC no. 246-585-8 Development codes BAS 351H (BASF)
bentazone-sodium
CAS RN [50723-80-3], formerly [92309-31-4]
PHYSICAL CHEMISTRY
bentazone
Composition ³96% pure. Mol. wt. 240.3 M.f. C10H12N2O3S Form Colourless crystals. M.p. 138 °C V.p. 0.17 mPa (20 °C) KOW logP = 0.77 (pH 5), -0.46 (pH 7), -0.55 (pH 9) Henry 7.167 ´ 10-5 Pa m3 mol-1 (calc.) S.g./density 1.41 (20 °C) Solubility In water 570 mg/l (pH 7, 20 ºC). In acetone 1387, methanol 1061, ethyl acetate 582, dichloromethane 206, n-heptane 0.5 ´ 10-3 (all in g/l, 20 ºC). Stability Very resistant to hydrolysis in both acidic and alkaline media. Decomposed by sunlight. pKa 3.3 (24 ºC)
bentazone-sodium
Mol. wt. 262.3 M.f. C10H11N2NaO3S Solubility In water 2.3 ´ 106 ppm (US EPA 1988).
COMMERCIALISATION
History Herbicide reported by A. Fischer (Proc. Br. Weed Control Conf., 9th, 1968, 2, 1042). Introduced by BASF AG in 1972. Patents US 3708277; DE 1542836 Manufacturers BASF; CAC; High Kite; Q.E.A.C.A.
APPLICATIONS
Biochemistry Photosynthetic electron transport inhibitor at the photosystem II receptor site. Mode of action Selective contact herbicide, absorbed mainly by the foliage, with very little translocation, but also absorbed by the roots, with translocation acropetally in the xylem. Uses A contact herbicide controlling Anthemis, Chamomilla and Matricaria spp., Chrysanthemum segetum, Galium aparine, Lapsana communis and Stellaria media in winter and spring cereals, at 1.0-2.2 kg/ha. Other crops include peanuts, maize, peas, Phaseolus beans, rice (Cyperus difformis, C. esculentus, C. serotinus, Monochoria vaginalis, Sagittaria pygmaea, S. sagittifolia, Alisma and Commelina spp., Scirpus maritimus and S. mucronatus) and soya beans (Abutilon theophrasti, Capsella bursa-pastoris, Cyperus esculentus, Datura stramonium, Helianthus spp., Polygonum spp., Portulaca spp., Sida spinosa, Ambrosia spp., Sinapis arvensis and Xanthium spp.). Formulation types SL.
bentazone
Selected products: 'Benta' (Sanonda)
bentazone-sodium
Selected products: 'Basagran' (BASF); mixtures: 'Doble' (+ acifluorfen-sodium) (Latin America) (BASF); 'Galaxy' (+ acifluorfen-sodium) (BASF); 'Storm' (+ acifluorfen-sodium) (BASF); 'Clincher Bas' (+ cyhalofop-butyl) (Dow AgroSciences)
OTHER PRODUCTS
bentazone
'Basamais' (BASF); 'Adagio' (Sipcam Phyteurop); 'Pilargran' (Pilarquim) mixtures: 'Basagran DP' (+ dichlorprop-dimethylammonium) (BASF); 'Extoll' (+ bromoxynil) (diolamine salts) (BASF); 'Impuls' (+ pendimethalin) (BASF); 'Archer' (+ MCPA+ MCPB) (Headland) Discontinued products mixtures: 'Clean Sweep' * (+ imazethapyr) (BASF); 'Dribble' * (+ dimefuron) (Feinchemie Schwebda); 'Topshot' * (+ cyanazine+ 2,4-DB) (Cyanamid)
bentazone-sodium
'Basagran Forte' (BASF) mixtures: 'Acumen' (+ MCPA-sodium+ MCPB-sodium) (MCPA may be as potassium or dimethylammonium salts) (BASF); 'Artett' (+ terbuthylazine) (BASF); 'Conclude B' (+ acifluorfen-sodium) (BASF); 'Conclude Ultra' (+ sethoxydim+ acifluorfen-sodium) (BASF); 'Conclude Xtra' (+ clethodim+ acifluorfen-sodium) (BASF); 'Laddok' (+ atrazine) (BASF, Sipcam); 'Pulsar' (+ MCPB-sodium) (BASF); 'Rezult' (+ sethoxydim) (BASF); 'Cambio' (+ dicamba-sodium) (De Sangosse) Discontinued products mixtures: 'Faster' * (+ fomesafen-sodium) (BASF); 'Manifest B' * (+ acifluorfen-sodium) (BASF)
ANALYSIS
Product analysis by rplc with u.v. detection (AOAC Methods, 17th Ed., 993.02; CIPAC Handbook, 1985, 1C, 1973) or by glc of a derivative. Residues determined by glc of a derivative with ECD (BBA Kurzfassungen zur Analytik von Pflanzenschutzmitteln in Wasser, 1989, 2, Auflage S. 42). In drinking water, by conversion to methyl ester with diazomethane, then glc with ECD (AOAC Methods, 17th Ed., 992.32). Details available from BASF.
MAMMALIAN TOXICOLOGY
Reviews FAO/WHO 83, 85, 86 (see part 2 of the Bibliography). Oral Acute oral LD50 for rats >1000, dogs >500, rabbits 750, cats 500 mg/kg. Skin and eye Acute percutaneous LD50 for rats >2500 mg/kg. Moderately irritating to skin and eyes (rabbits). Inhalation LC50 (4 h) for rats >5.1 mg/l air. NOEL (1 y) for dogs 13.1 mg/kg b.w.; (2 y) for rats 10 mg/kg b.w.; (90 d) for rats 25, dogs 10 mg/kg b.w.; (78 w) for mice 12 mg/kg b.w. ADI (JMPR) 0.1 mg/kg [1998, 1999]. Water GV 300 mg/l (based on ADI). Toxicity class WHO (a.i.) III; EPA (formulation) III EC classification Xi; R36| R43| R52, R53
ECOTOXICOLOGY
bentazone
Birds Acute oral LD50 for bobwhite quail 1140 mg/kg. Dietary LC50 for bobwhite quail and mallard ducks >5000 ppm. Fish LC50 (96 h) for rainbow trout and bluegill sunfish >100 mg/l. Daphnia LC50 (48 h) 125 mg/l. Algae EC50 (72 h) for green algae (Ankistrodesmus) 47.3 mg/l. Bees Not toxic to bees; LD50 (oral) >100 mg/bee. Worms EC50 (14 d) >1000 mg/kg soil.
ENVIRONMENTAL FATE
Animals Metabolism studies in three different species showed that bentazone was only poorly metabolised, the parent compound being the predominant product. Only small amounts of hydroxylated bentazone metabolites could be detected. No conjugated products were found. Plants In plants, rapidly metabolised to derivatives of anthranilic acid, the principal metabolites being the 6- and 8-hydroxy derivatives, which are conjugated to sugars, forming glycosides. Soil/Environment In soil, short-lived hydroxy compounds are first formed, which rapidly undergo further degradation (R. Huber & S. Otto in Rev. Environ. Contam. Toxicol., 137, 111-134). In sunlight, bentazone undergoes rapid degradation, ultimately to CO2. It has low soil persistence: in freshly collected field soils, aerobic DT50 (lab., 20 ºC) was 14 d. In lab. degradation studies, with soils of proven biological activity, DT50 (average) was 17.8 d; DT50 (field, average) was c. 12 d; corresponding DT90 was 44 d. Koc 13.3-176 ml/g (average 42 ml/g), indicating mobility. However, when used according to good agricultural practice, bentazone is degraded more quickly than it can leach; in all lysimeter studies, average annual leachates contained <0.1 mg/l.
|